On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo
- PMID: 19226371
- PMCID: PMC3666345
- DOI: 10.1111/j.1471-4159.2009.05893.x
On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo
Abstract
In the mammalian brain, kynurenine aminotransferase II (KAT II) and kynurenine 3-monooxygenase (KMO), key enzymes of the kynurenine pathway (KP) of tryptophan degradation, form the neuroactive metabolites kynurenic acid (KYNA) and 3-hydroxykynurenine (3-HK), respectively. Although physically segregated, both enzymes use the pivotal KP metabolite l-kynurenine as a substrate. We studied the functional consequences of this cellular compartmentalization in vivo using two specific tools, the KAT II inhibitor BFF 122 and the KMO inhibitor UPF 648. The acute effects of selective KAT II or KMO inhibition were studied using a radiotracing method in which the de novo synthesis of KYNA, and of 3-HK and its downstream metabolite quinolinic acid (QUIN), is monitored following an intrastriatal injection of (3)H-kynurenine. In naïve rats, intrastriatal BFF 122 decreased newly formed KYNA by 66%, without influencing 3-HK or QUIN production. Conversely, UPF 648 reduced 3-HK synthesis (by 64%) without affecting KYNA formation. Similar, selective effects of KAT II and KMO inhibition were observed when the inhibitors were applied acutely together with the excitotoxin QUIN, which impairs local KP metabolism. Somewhat different effects of KMO (but not KAT II) inhibition were obtained in rats that had received an intrastriatal QUIN injection 7 days earlier. In these neuron-depleted striata, UPF 648 not only decreased both 3-HK and QUIN production (by 77% and 66%, respectively) but also moderately raised KYNA synthesis (by 27%). These results indicate a remarkable functional segregation of the two pathway branches in the brain, boding well for the development of selective KAT II or KMO inhibitors for cognitive enhancement and neuroprotection, respectively.
Figures

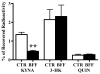


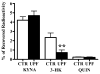



References
-
- Amori L. PhD Dissertation. University of Perugia; 2003. Disegno e sintesi di nuovi potenziali inibitori della chinurenina 3-idrossilasi, enzima della via metabolica delle chinurenine.
-
- Alkondon M, Pereira EF, Yu P, Arruda EZ, Almeida LE, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, Tagle DA, Albuquerque EX. Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–4648. - PMC - PubMed
-
- Belladonna ML, Puccetti P, Orabona C, Fallarino F, Vacca C, Volpi C, Gizzi S, Pallotta MT, Fioretti MC, Grohmann U. Immunosuppression via tryptophan catabolism: the role of kynurenine pathway enzymes. Transplantation. 2007;84:S17–S20. - PubMed
-
- Bender DA, McCreanor GM. The preferred route of kynurenine metabolism in the rat. Biochim Biophys Acta. 1982;717:56–60. - PubMed
-
- Bergeron R, Wu H-Q, Potter MC, Guidetti P, Schwarcz R. Mice with reduced brain kynurenic acid levels have high extracellular glutamate and show enhanced LTP in the hippocampus. Soc Neurosci Abstr. 2007;32:807.15.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

