Evolution of the sugar receptors in insects
- PMID: 19226470
- PMCID: PMC2667405
- DOI: 10.1186/1471-2148-9-41
Evolution of the sugar receptors in insects
Abstract
Background: Perception of sugars is an invaluable ability for insects which often derive quickly accessible energy from these molecules. A distinctive subfamily of eight proteins within the gustatory receptor (Gr) family has been identified as sugar receptors (SRs) in Drosophila melanogaster (Gr5a, Gr61a, and Gr64a-f). We examined the evolution of these SRs within the 12 available Drosophila genome sequences, as well as three mosquito, two moth, and beetle, bee, and wasp genome sequences.
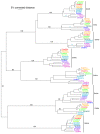
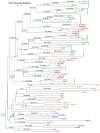
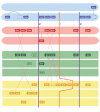
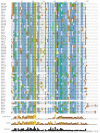
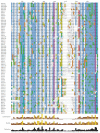
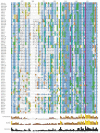
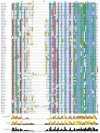
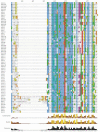
Results: While most Drosophila species retain all eight genes, we find that the three Drosophila subgenus species have lost Gr64d, while D. grimshawi and the D. pseudoobscura/persimilis sibling species have also lost Gr5a function. The entire Gr64 gene complex was also duplicated in the D. grimshawi lineage, but only one potentially functional copy of each gene has been retained. The numbers of SRs range from two in the hymenopterans Apis mellifera and Nasonia vitripennis to 16 in the beetle Tribolium castaneum. An unusual aspect is the evolution of a novel exon from intronic sequence in an expanded set of four SRs in Bombyx mori (BmGr5-8), which appears to be the first example of such exonization in insects. Twelve intron gains and 63 losses are inferred within the SR family.
Conclusion: Examination of the SRs in these fly, mosquito, moth, beetle, and hymenopteran genome sequences reveals that they appear to have originated independently from single ancestral genes within the dipteran and coleopteran lineages, and two genes in the lepidopteran and hymenopteran lineages. The origin of the insect SRs will eventually be illuminated by additional basal insect and arthropod genome sequences.
Figures
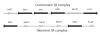
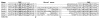



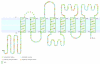
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

