High resolution preparation of monocyte-derived macrophages (MDM) protein fractions for clinical proteomics
- PMID: 19228399
- PMCID: PMC2649903
- DOI: 10.1186/1477-5956-7-4
High resolution preparation of monocyte-derived macrophages (MDM) protein fractions for clinical proteomics
Abstract
Background: Macrophages are involved in a number of key physiological processes and complex responses such as inflammatory, immunological, infectious diseases and iron homeostasis. These cells are specialised for iron storage and recycling from senescent erythrocytes so they play a central role in the fine tuning of iron balancing and distribution. The comprehension of the many physiological responses of macrophages implies the study of the related molecular events. To this regard, proteomic analysis, is one of the most powerful tools for the elucidation of the molecular mechanisms, in terms of changes in protein expression levels.
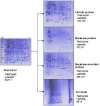
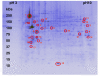
Results: Our aim was to optimize a protocol for protein fractionation and high resolution mapping using human macrophages for clinical studies. We exploited a fractionation protocol based on the neutral detergent Triton X-114. The 2D maps of the fractions obtained showed high resolution and a good level of purity. Western immunoblotting and mass spectrometry (MS/MS analysis) indicated no fraction cross contamination. On 2D-PAGE mini gels (7 x 8 cm) we could count more than five hundred protein spots, substantially increasing the resolution and the number of detectable proteins for the macrophage proteome. The fractions were also evaluated, with preliminary experiments, using Surface Enhanced Laser Desorption Ionization Time of Flight Mass Spectrometry (SELDI-TOF-MS).
Conclusion: This relatively simple method allows deep investigation into macrophages proteomics producing discrete and accurate protein fractions, especially membrane-associated and integral proteins. The adapted protocol seems highly suitable for further studies of clinical proteomics, especially for the elucidation of the molecular mechanisms controlling iron homeostasis in normal and disease conditions.
Figures


Similar articles
-
Material surfaces affect the protein expression patterns of human macrophages: A proteomics approach.J Biomed Mater Res A. 2007 Mar 15;80(4):895-908. doi: 10.1002/jbm.a.30967. J Biomed Mater Res A. 2007. PMID: 17072854 Clinical Trial.
-
Murine macrophages response to iron.J Proteomics. 2012 Dec 5;76 Spec No.:10-27. doi: 10.1016/j.jprot.2012.07.018. Epub 2012 Jul 23. J Proteomics. 2012. PMID: 22835775
-
A SELDI mass spectrometry study of experimental autoimmune encephalomyelitis: sample preparation, reproducibility, and differential protein expression patterns.Proteome Sci. 2013 May 1;11(1):19. doi: 10.1186/1477-5956-11-19. Proteome Sci. 2013. PMID: 23635033 Free PMC article.
-
[Research progress in SELDI-TOF MS and its clinical applications].Sheng Wu Gong Cheng Xue Bao. 2006 Nov;22(6):871-6. doi: 10.1016/s1872-2075(06)60061-7. Sheng Wu Gong Cheng Xue Bao. 2006. PMID: 17168305 Free PMC article. Review.
-
Surface-enhanced laser desorption ionization time-of-flight mass spectrometry (SELDI TOF-MS) and ProteinChip technology in proteomics research.Pathol Res Pract. 2004;200(2):83-94. doi: 10.1016/j.prp.2004.01.010. Pathol Res Pract. 2004. PMID: 15237917 Review.
Cited by
-
Molecular Consequences of Proprotein Convertase 1/3 (PC1/3) Inhibition in Macrophages for Application to Cancer Immunotherapy: A Proteomic Study.Mol Cell Proteomics. 2015 Nov;14(11):2857-77. doi: 10.1074/mcp.M115.052480. Epub 2015 Sep 1. Mol Cell Proteomics. 2015. PMID: 26330543 Free PMC article.
-
Disruption of proprotein convertase 1/3 (PC1/3) expression in mice causes innate immune defects and uncontrolled cytokine secretion.J Biol Chem. 2012 Apr 27;287(18):14703-17. doi: 10.1074/jbc.M111.323220. Epub 2012 Mar 6. J Biol Chem. 2012. PMID: 22396549 Free PMC article.
-
In-Depth Proteomic Characterization of Classical and Non-Classical Monocyte Subsets.Proteomes. 2018 Feb 5;6(1):8. doi: 10.3390/proteomes6010008. Proteomes. 2018. PMID: 29401756 Free PMC article.
-
Quantitative proteomic analysis of host--pathogen interactions: a study of Acinetobacter baumannii responses to host airways.BMC Genomics. 2015 May 30;16(1):422. doi: 10.1186/s12864-015-1608-z. BMC Genomics. 2015. PMID: 26025090 Free PMC article.
References
-
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. - PubMed
-
- De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72–81. - PubMed
-
- Hansson GK. Inflammation and immune response in atherosclerosis. Curr Atheroscler Rep. 1999;1:150–155. - PubMed
-
- Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. - PubMed
LinkOut - more resources
Full Text Sources

