Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts
- PMID: 19228708
- PMCID: PMC2675932
- DOI: 10.1093/cvr/cvp056
Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts
Abstract
Aims: Following injury, fibroblasts transform into myofibroblasts and produce extracellular matrix (ECM). Excess production of ECM associated with cardiac fibrosis severely inhibits cardiac function. Sphingosine-1-phosphate (S1P), a bioactive lysophospholipid, regulates the function of numerous cell types. In this study, we determined the role of S1P in promoting pro-fibrotic actions of cardiac fibroblasts (CFs).
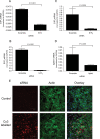
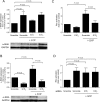
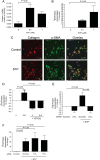
Methods and results: S1P-mediated effects on myofibroblast transformation, collagen production, and cross-talk with transforming growth factor-beta (TGF-beta) using mouse CF were examined. S1P increased alpha-smooth muscle actin (a myofibroblast marker) and collagen expression in a S1P2 receptor- and Rho kinase-dependent manner. TGF-beta increased sphingosine kinase 1 (SphK1; the enzyme responsible for S1P production) expression and activity. TGF-beta-stimulated collagen production was inhibited by SphK1 or S1P2 siRNA, a SphK inhibitor, and an anti-S1P monoclonal antibody.
Conclusion: These findings suggest that TGF-beta-stimulated collagen production in CF involves 'inside-out' S1P signalling whereby S1P produced intracellularly by SphK1 can be released and act in an autocrine/paracrine fashion to activate S1P2 and increase collagen production.
Figures



References
-
- Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. - PubMed
-
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. - PubMed
-
- Sun Y, Kiani MF, Postlethwaite AE, Weber KT. Infarct scar as living tissue. Basic Res Cardiol. 2002;97:343–347. - PubMed
-
- Long CS, Brown RD. The cardiac fibroblast, another therapeutic target for mending the broken heart? J Mol Cell Cardiol. 2002;34:1273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

