Combining the endoplasmic reticulum stress-inducing agents bortezomib and fenretinide as a novel therapeutic strategy for metastatic melanoma
- PMID: 19228725
- PMCID: PMC2917762
- DOI: 10.1158/1078-0432.CCR-08-2150
Combining the endoplasmic reticulum stress-inducing agents bortezomib and fenretinide as a novel therapeutic strategy for metastatic melanoma
Abstract
Purpose: Single-agent chemotherapy is largely the treatment of choice for systemic therapy of metastatic melanoma, but survival rates are low, and novel adjuvant and systemic therapies are urgently required. Endoplasmic reticulum (ER) stress is a potential therapeutic target, and two relatively new drugs, fenretinide and bortezomib (Velcade), each acting via different cellular mechanisms, induce ER stress leading to apoptosis in melanoma cells. The aim of this study was to test the hypothesis that apoptosis of melanoma cells may be increased by combining clinically achievable concentrations of fenretinide and bortezomib.
Experimental design: Three human melanoma cell lines were used to assess changes in viability and the induction of apoptosis in response to fenretinide, bortezomib, or both drugs together. A s.c. xenograft model was used to test responses in vivo.
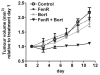
Results: Fenretinide and bortezomib synergistically decreased viability and increased apoptosis in all three melanoma lines at clinically achievable concentrations. This was also reflected by increased expression of GADD153, a marker of ER stress-induced apoptosis. In vivo, fenretinide in combination with bortezomib gave a marked reduction in xenograft tumor volume and an increase in apoptosis compared with fenretinide or bortezomib alone. The cell cycle stage of tumor cells in vivo were similar to that predicted from the effects of each drug or the combination in vitro.
Conclusions: These results suggest that fenretinide and bortezomib, both of which are available in clinical formulation, warrant clinical evaluation as a combination therapy for metastatic melanoma.
Figures



References
-
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous Melanoma. Lancet. 2005;365:687–701. - PubMed
-
- Rass K, Tilgen W. Treatment of melanoma and non melanoma skin cancer. Adv Exp Med Biol. 2008;624:296–318. - PubMed
-
- Atallah E, Flaherty L. Treatment of metastatic maliganant melanoma. Current Treatment Options in Oncology. 2005;6:185–93. - PubMed
-
- Quirt I, Verma S, Petrella T, Bak K, Charette M. Temozolomide for the treatment of metastatic melanoma: a systemic review. Oncologist. 2007;12:1114–23. - PubMed
-
- Xiao C, Yang BF, Song JH, Schulman H, Li L, Hao C. Inhibition of CaMKII-mediated c-FLIP expression sensitizes malignant melanoma cells to TRAIL-induced apoptosis. Exp Cell Res. 2005;304:244–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

