Preclinical studies in support of defibrotide for the treatment of multiple myeloma and other neoplasias
- PMID: 19228727
- PMCID: PMC4401498
- DOI: 10.1158/1078-0432.CCR-08-1270
Preclinical studies in support of defibrotide for the treatment of multiple myeloma and other neoplasias
Abstract
Purpose of the study: Defibrotide, an orally bioavailable polydisperse oligonucleotide, has promising activity in hepatic veno-occlusive disease, a stem cell transplantation-related toxicity characterized by microangiopathy. The antithrombotic properties of defibrotide and its minimal hemorrhagic risk could serve for treatment of cancer-associated thrombotic complications. Given its cytoprotective effect on endothelium, we investigated whether defibrotide protects tumor cells from cytotoxic antitumor agents. Further, given its antiadhesive properties, we evaluated whether defibrotide modulates the protection conferred to multiple myeloma cells by bone marrow stromal cells.
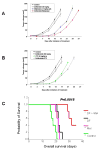
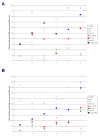
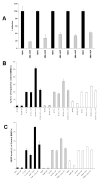
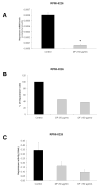
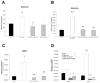
Methods-results: Defibrotide lacks significant single-agent in vitro cytotoxicity on multiple myeloma or solid tumor cells and does not attenuate their in vitro response to dexamethasone, bortezomib, immunomodulatory thalidomide derivatives, and conventional chemotherapeutics, including melphalan and cyclophosphamide. Importantly, defibrotide enhances in vivo chemosensitivity of multiple myeloma and mammary carcinoma xenografts in animal models. In cocultures of multiple myeloma cells with bone marrow stromal cells in vitro, defibrotide enhances the multiple myeloma cell sensitivity to melphalan and dexamethasone, and decreases multiple myeloma-bone marrow stromal cell adhesion and its sequelae, including nuclear factor-kappaB activation in multiple myeloma and bone marrow stromal cells, and associated cytokine production. Moreover, defibrotide inhibits expression and/or function of key mediators of multiple myeloma interaction with bone marrow stromal cell and endothelium, including heparanase, angiogenic cytokines, and adhesion molecules.
Conclusion: Defibrotide's in vivo chemosensitizing properties and lack of direct in vitro activity against tumor cells suggest that it favorably modulates antitumor interactions between bone marrow stromal cells and endothelia in the tumor microenvironment. These data support clinical studies of defibrotide in combination with conventional and novel therapies to potentially improve patient outcome in multiple myeloma and other malignancies.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
C. Echart, M. Distaso, M. Iacobelli, employees, Gentium. C. Rouleau B. Teicher, employees, Genzyme. M. Iacobelli, ownership interest, Gentium. B. Teicher, ownership interest, Genzyme. P. Richardson, commercial research grant, and advisory board, Gentium; honoraria, Celgene and Millennium. A. Palumbo, honoraria and advisory board, Celgene, and Johnson & Johnson. C. Mitsiades, honoraria, Millennium, Novartis and Pharmion. K. Anderson, board Gentium; advisory board and honoraria, Celgene, Millennium, Novartis, Johnson and Johnson
Figures

References
-
- Lee AY. Management of thrombosis in cancer: primary prevention and secondary prophylaxis. Br J Haematol. 2005;128:291–302. - PubMed
-
- Knight R, DeLap RJ, Zeldis JB. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354:2079–80. - PubMed
-
- Pescador R, Porta R, Ferro L. An integrated view of the activities of defibrotide. Semin Thromb Hemost. 1996;22 (Suppl 1):71–5. - PubMed
-
- Eissner G, Multhoff G, Gerbitz A, et al. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide. Blood. 2002;100:334–40. - PubMed
-
- Palmer KJ, Goa KL. Defibrotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in vascular disorders. Drugs. 1993;45:259–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

