Murine dendritic cell antigen-presenting cell function is not altered by burn injury
- PMID: 19228816
- PMCID: PMC2669406
- DOI: 10.1189/jlb.0408257
Murine dendritic cell antigen-presenting cell function is not altered by burn injury
Abstract
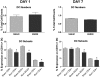
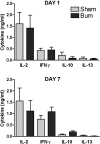
Severe injury disrupts normal immune regulation causing a transient hyperinflammatory reaction and suppressed adaptive immune function. This report addresses the potential contribution of dendritic cells (DC) to changes in adaptive immune function after injury by specifically measuring injury-induced changes in splenic DC numbers and subsets, cell-surface markers, TLR responses, and APC function. Using a mouse burn injury model, we found that injury did not markedly alter the relative percentage of lymphoid, myeloid, or plasmacytoid DC in the spleens of burn-injured mice. Moreover, we did not observe a significant reduction in cell-surface expression of several major costimulatory molecules, CD40, CD80, CD86, programmed death 1 ligand, ICOS ligand, and B7-H3, on DC. Instead, we observed increased cell-surface expression of CD86 at 1 day after injury with no significant changes in costimulatory molecule expression at 7 days after injury, suggesting that burn injury causes an early activation of DC. In addition, injury did not suppress DC reactivity to TLR2, TLR4, or TLR9 agonists. Most important, DC prepared from injured mice were able to present peptide antigen to naive OTII TCR transgenic CD4+ T cells as efficiently and effectively as DC from sham-injured mice. We also found that CD4 T cells stimulated with antigen presented by DC from sham or burn mice showed similar levels of IL-2, IFN-gamma, IL-10, and IL-13 production. Taken together, these findings support the conclusion that DC do not acquire a suppressive phenotype following severe injury in mice.
Figures

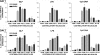
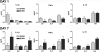
References
-
- Smith J W, Gamelli R L, Jones S B, Shankar R. Immunologic responses to critical injury and sepsis. J Intensive Care Med. 2006;21:160–172. - PubMed
-
- Wilkinson R A, Fishman J A. Effect of thermal injury with Pseudomonas aeruginosa infection on pulmonary and systemic bacterial clearance. J Trauma. 1999;47:912–917. - PubMed
-
- Kobayashi M, Kobayashi H, Herndon D N, Pollard R B, Suzuki F. Burn-associated Candida albicans infection caused by CD30+ type 2 T cells. J Leukoc Biol. 1998;63:723–731. - PubMed
-
- Kobayashi H, Kobayashi M, Utsunomiya T, Herndon D N, Pollard R B, Suzuki F. Therapeutic protective effects of IL-12 combined with soluble IL-4 receptor against established infections of herpes simplex virus type 1 in thermally injured mice. J Immunol. 1999;162:7148–7154. - PubMed
-
- Zedler S, Bone R C, Baue A E, von Donnersmarck G H, Faist E. T-cell reactivity and its predictive role in immunosuppression after burns. Crit Care Med. 1999;27:66–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

