A HaemAtlas: characterizing gene expression in differentiated human blood cells
- PMID: 19228925
- PMCID: PMC2680378
- DOI: 10.1182/blood-2008-06-162958
A HaemAtlas: characterizing gene expression in differentiated human blood cells
Abstract
Hematopoiesis is a carefully controlled process that is regulated by complex networks of transcription factors that are, in part, controlled by signals resulting from ligand binding to cell-surface receptors. To further understand hematopoiesis, we have compared gene expression profiles of human erythroblasts, megakaryocytes, B cells, cytotoxic and helper T cells, natural killer cells, granulocytes, and monocytes using whole genome microarrays. A bioinformatics analysis of these data was performed focusing on transcription factors, immunoglobulin superfamily members, and lineage-specific transcripts. We observed that the numbers of lineage-specific genes varies by 2 orders of magnitude, ranging from 5 for cytotoxic T cells to 878 for granulocytes. In addition, we have identified novel coexpression patterns for key transcription factors involved in hematopoiesis (eg, GATA3-GFI1 and GATA2-KLF1). This study represents the most comprehensive analysis of gene expression in hematopoietic cells to date and has identified genes that play key roles in lineage commitment and cell function. The data, which are freely accessible, will be invaluable for future studies on hematopoiesis and the role of specific genes and will also aid the understanding of the recent genome-wide association studies.
Figures
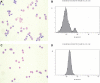

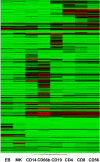


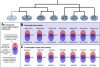

Comment in
-
Comparative genomics: fishing nets hemostatic catch.Blood. 2009 May 7;113(19):4479-80. doi: 10.1182/blood-2009-02-203117. Blood. 2009. PMID: 19423736 No abstract available.
References
-
- Zola H, Swart B, Nicholson I, et al. CD molecules 2005: human cell differentiation molecules. Blood. 2005;106:3123–3126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

