Recent speciation associated with the evolution of selfing in Capsella
- PMID: 19228944
- PMCID: PMC2664025
- DOI: 10.1073/pnas.0807679106
Recent speciation associated with the evolution of selfing in Capsella
Abstract
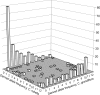
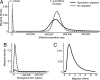
The evolution from outcrossing to predominant self-fertilization represents one of the most common transitions in flowering plant evolution. This shift in mating system is almost universally associated with the "selfing syndrome," characterized by marked reduction in flower size and a breakdown of the morphological and genetic mechanisms that prevent self-fertilization. In general, the timescale in which these transitions occur, and the evolutionary dynamics associated with the evolution of the selfing syndrome are poorly known. We investigated the origin and evolution of selfing in the annual plant Capsella rubella from its self-incompatible, outcrossing progenitor Capsella grandiflora by characterizing multilocus patterns of DNA sequence variation at nuclear genes. We estimate that the transition to selfing and subsequent geographic expansion have taken place during the past 20,000 years. This transition was probably associated with a shift from stable equilibrium toward a near-complete population bottleneck causing a major reduction in effective population size. The timing and severe founder event support the hypothesis that selfing was favored during colonization as new habitats emerged after the last glaciation and the expansion of agriculture. These results suggest that natural selection for reproductive assurance can lead to major morphological evolution and speciation on relatively short evolutionary timescales.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

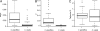
References
-
- Charlesworth D. Evolution of plant breeding systems. Curr Biol. 2006;16:R726–R735. - PubMed
-
- Stebbins GL. Variation and Evolution in Plants. New York: Columbia Univ Press; 1950.
-
- Barrett SCH. The evolution of plant sexual diversity. Nat Rev. 2002;3:274–284. - PubMed
-
- Fisher RA. Average excess and average effect of a gene substitution. Ann Eugen. 1941;11:53–63.
-
- Darwin CR. The Effects of Cross and Self-fertilization in the Vegetable Kingdom. London: John Murray; 1876.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

