Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels
- PMID: 19228957
- PMCID: PMC2667244
- DOI: 10.1523/JNEUROSCI.4531-08.2009
Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels
Abstract
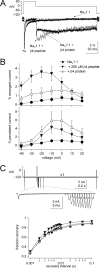
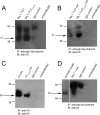
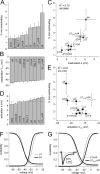
The beta subunits of voltage-gated Na channels (Scnxb) regulate the gating of pore-forming alpha subunits, as well as their trafficking and localization. In heterologous expression systems, beta1, beta2, and beta3 subunits influence inactivation and persistent current in different ways. To test how the beta4 protein regulates Na channel gating, we transfected beta4 into HEK (human embryonic kidney) cells stably expressing Na(V)1.1. Unlike a free peptide with a sequence from the beta4 cytoplasmic domain, the full-length beta4 protein did not block open channels. Instead, beta4 expression favored open states by shifting activation curves negative, decreasing the slope of the inactivation curve, and increasing the percentage of noninactivating current. Consequently, persistent current tripled in amplitude. Expression of beta1 or chimeric subunits including the beta1 extracellular domain, however, favored inactivation. Coexpressing Na(V)1.1 and beta4 with beta1 produced tiny persistent currents, indicating that beta1 overcomes the effects of beta4 in heterotrimeric channels. In contrast, beta1(C121W), which contains an extracellular epilepsy-associated mutation, did not counteract the destabilization of inactivation by beta4 and also required unusually large depolarizations for channel opening. In cultured hippocampal neurons transfected with beta4, persistent current was slightly but significantly increased. Moreover, in beta4-expressing neurons from Scn1b and Scn1b/Scn2b null mice, entry into inactivated states was slowed. These data suggest that beta1 and beta4 have antagonistic roles, the former favoring inactivation, and the latter favoring activation. Because increased Na channel availability may facilitate action potential firing, these results suggest a mechanism for seizure susceptibility of both mice and humans with disrupted beta1 subunits.
Figures





References
-
- Abriel H, Kass RS. Regulation of the voltage-gated cardiac sodium channel Nav1.5 by interacting proteins. Trends Cardiovasc Med. 2005;15:35–40. - PubMed
-
- Afshari FS, Ptak K, Khaliq ZM, Grieco TM, Slater NT, McCrimmon DR, Raman IM. Resurgent Na currents in four classes of neurons in the cerebellum. J Neurophysiol. 2004;92:2831–2843. - PubMed
-
- Annesi G, Gambardella A, Carrideo S, Incorpora G, Labate A, Pasqua AA, Civitelli D, Polizzi A, Annesi F, Spadafora P, Tarantino P, Cirò Candiano IC, Romeo N, De Marco EV, Ventura P, LePiane E, Zappia M, Aguglia U, Pavone L, Quattrone A. Two novel SCN1A missense mutations in generalized epilepsy with febrile seizures plus. Epilepsia. 2003;44:1257–1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
