Interdependent encoding of pitch, timbre, and spatial location in auditory cortex
- PMID: 19228960
- PMCID: PMC2663390
- DOI: 10.1523/JNEUROSCI.4755-08.2009
Interdependent encoding of pitch, timbre, and spatial location in auditory cortex
Abstract
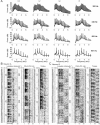
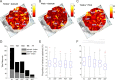
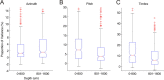
Because we can perceive the pitch, timbre, and spatial location of a sound source independently, it seems natural to suppose that cortical processing of sounds might separate out spatial from nonspatial attributes. Indeed, recent studies support the existence of anatomically segregated "what" and "where" cortical processing streams. However, few attempts have been made to measure the responses of individual neurons in different cortical fields to sounds that vary simultaneously across spatial and nonspatial dimensions. We recorded responses to artificial vowels presented in virtual acoustic space to investigate the representations of pitch, timbre, and sound source azimuth in both core and belt areas of ferret auditory cortex. A variance decomposition technique was used to quantify the way in which altering each parameter changed neural responses. Most units were sensitive to two or more of these stimulus attributes. Although indicating that neural encoding of pitch, location, and timbre cues is distributed across auditory cortex, significant differences in average neuronal sensitivity were observed across cortical areas and depths, which could form the basis for the segregation of spatial and nonspatial cues at higher cortical levels. Some units exhibited significant nonlinear interactions between particular combinations of pitch, timbre, and azimuth. These interactions were most pronounced for pitch and timbre and were less commonly observed between spatial and nonspatial attributes. Such nonlinearities were most prevalent in primary auditory cortex, although they tended to be small compared with stimulus main effects.
Figures


References
-
- Barrett DJ, Hall DA. Response preferences for “what” and “where” in human non-primary auditory cortex. Neuroimage. 2006;32:968–977. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
