The cochaperone BAG2 sweeps paired helical filament- insoluble tau from the microtubule
- PMID: 19228967
- PMCID: PMC2768429
- DOI: 10.1523/JNEUROSCI.4660-08.2009
The cochaperone BAG2 sweeps paired helical filament- insoluble tau from the microtubule
Abstract
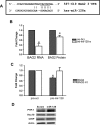
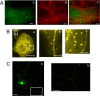
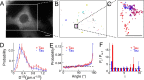
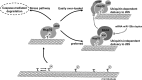
Tau inclusions are a prominent feature of many neurodegenerative diseases including Alzheimer's disease. Their accumulation in neurons as ubiquitinated filaments suggests a failure in the degradation limb of the Tau pathway. The components of a Tau protein triage system consisting of CHIP/Hsp70 and other chaperones have begun to emerge. However, the site of triage and the master regulatory elements are unknown. Here, we report an elegant mechanism of Tau degradation involving the cochaperone BAG2. The BAG2/Hsp70 complex is tethered to the microtubule and this complex can capture and deliver Tau to the proteasome for ubiquitin-independent degradation. This complex preferentially degrades Sarkosyl insoluble Tau and phosphorylated Tau. BAG2 levels in cells are under the physiological control of the microRNA miR-128a, which can tune paired helical filament Tau levels in neurons. Thus, we propose that ubiquitinated Tau inclusions arise due to shunting of Tau degradation toward a less efficient ubiquitin-dependent pathway.
Figures



References
-
- Ackmann M, Wiech H, Mandelkow E. Nonsaturable binding indicates clustering of tau on the microtubule surface in a paired helical filament-like conformation. J Biol Chem. 2000;275:30335–30343. - PubMed
-
- Alberti S, Demand J, Esser C, Emmerich N, Schild H, Hohfeld J. Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J Biol Chem. 2002;277:45920–45927. - PubMed
-
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
