The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse
- PMID: 19228970
- PMCID: PMC6666350
- DOI: 10.1523/JNEUROSCI.4997-08.2009
The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse
Abstract
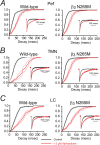
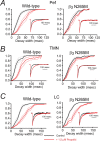
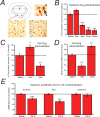
The GABA(A) receptor has been identified as the single most important target for the intravenous anesthetic propofol. How effects at this receptor are then translated into a loss of consciousness, however, remains a mystery. One possibility is that anesthetics act on natural sleep pathways. Here, we test this hypothesis by exploring the anesthetic sensitivities of GABAergic synaptic currents in three specific brain nuclei that are known to be involved in sleep. Using whole-cell electrophysiology, we have recorded GABAergic IPSCs from the tuberomammillary nucleus (TMN), the perifornical area (Pef), and the locus ceruleus (LC) in brain slices from both wild-type mice and mice that carry a specific mutation in the GABA(A) receptor beta(3) subunit (N265M), which greatly reduces their sensitivity to propofol, but not to the neurosteroid alphaxalone. We find that this in vivo pattern of anesthetic sensitivity is mirrored in the hypothalamic TMN and Pef nuclei, consistent with their role as direct anesthetic targets. In contrast, anesthetic sensitivity in the LC was unaffected by the beta(3)N265M mutation, ruling out this nucleus as a major target for propofol. In support of the hypothesis that orexinergic neurons in the Pef are involved in propofol anesthesia, we further show that these neurons are selectively inhibited by GABAergic drugs in vivo during anesthesia, and that a modulation in the activity of Pef neurons alone can affect loss of righting reflex. Overall, our results support the idea that GABAergic anesthetics such as propofol exert their effects, at least in part, by modulating hypothalamic sleep pathways.
Figures
Similar articles
-
GABAA receptors involved in sleep and anaesthesia: β1- versus β3-containing assemblies.Pflugers Arch. 2012 Jan;463(1):187-99. doi: 10.1007/s00424-011-0988-4. Epub 2011 Jul 7. Pflugers Arch. 2012. PMID: 21735059
-
Pharmacological properties of GABAA receptors in rat hypothalamic neurons expressing the epsilon-subunit.J Neurosci. 2005 Jan 5;25(1):88-95. doi: 10.1523/JNEUROSCI.3209-04.2005. J Neurosci. 2005. PMID: 15634770 Free PMC article.
-
The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway.Nat Neurosci. 2002 Oct;5(10):979-84. doi: 10.1038/nn913. Nat Neurosci. 2002. PMID: 12195434
-
General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal.Nat Rev Neurosci. 2008 May;9(5):370-86. doi: 10.1038/nrn2372. Nat Rev Neurosci. 2008. PMID: 18425091 Review.
-
Identification and characterization of anesthetic targets by mouse molecular genetics approaches.Can J Anaesth. 2011 Feb;58(2):178-90. doi: 10.1007/s12630-010-9414-1. Epub 2010 Dec 21. Can J Anaesth. 2011. PMID: 21174184 Free PMC article. Review.
Cited by
-
Dextroamphetamine (but Not Atomoxetine) Induces Reanimation from General Anesthesia: Implications for the Roles of Dopamine and Norepinephrine in Active Emergence.PLoS One. 2015 Jul 6;10(7):e0131914. doi: 10.1371/journal.pone.0131914. eCollection 2015. PLoS One. 2015. PMID: 26148114 Free PMC article.
-
Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine β-hydroxylase knockout mice.Anesthesiology. 2012 Nov;117(5):1006-17. doi: 10.1097/ALN.0b013e3182700ab9. Anesthesiology. 2012. PMID: 23042227 Free PMC article.
-
Neural Ensemble Fragmentation in the Anesthetized Drosophila Brain.J Neurosci. 2023 Apr 5;43(14):2537-2551. doi: 10.1523/JNEUROSCI.1657-22.2023. Epub 2023 Mar 3. J Neurosci. 2023. PMID: 36868857 Free PMC article.
-
Inactivation of the Tuberomammillary Nucleus by GABAA Receptor Agonist Promotes Slow Wave Sleep in Freely Moving Rats and Histamine-Treated Rats.Neurochem Res. 2017 Aug;42(8):2314-2325. doi: 10.1007/s11064-017-2247-3. Epub 2017 Apr 1. Neurochem Res. 2017. PMID: 28365867
-
GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness.J Neurosci. 2012 Sep 19;32(38):13062-75. doi: 10.1523/JNEUROSCI.2931-12.2012. J Neurosci. 2012. PMID: 22993424 Free PMC article.
References
-
- Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. - PubMed
-
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. - PubMed
-
- Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, Jones BE. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
