Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice
- PMID: 19228979
- PMCID: PMC6666325
- DOI: 10.1523/JNEUROSCI.5593-08.2009
Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice
Abstract
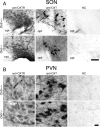
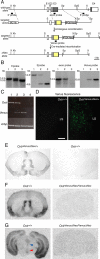
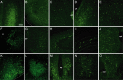
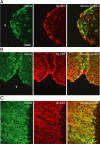
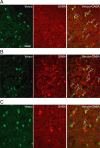
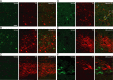
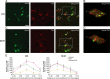
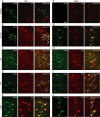
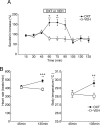
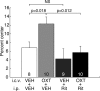
The oxytocin receptor has been implicated in the regulation of reproductive physiology as well as social and emotional behaviors. The neurochemical mechanisms by which oxytocin receptor modulates social and emotional behavior remains elusive, in part because of a lack of sensitive and selective antibodies for cellular localization. To more precisely characterize oxytocin receptor-expressing neurons within the brain, we generated an oxytocin receptor-reporter mouse in which part of the oxytocin receptor gene was replaced with Venus cDNA (a variant of yellow fluorescent protein). Examination of the Venus expression revealed that, in the raphe nuclei, about one-half of tryptophan hydroxylase-immunoreactive neurons were positive for Venus, suggesting a potential role for oxytocin in the modulation of serotonin release. Oxytocin infusion facilitated serotonin release within the median raphe nucleus and reduced anxiety-related behavior. Infusion of a 5-HT(2A/2C) receptor antagonist blocked the anxiolytic effect of oxytocin, suggesting that oxytocin receptor activation in serotonergic neurons mediates the anxiolytic effects of oxytocin. This is the first demonstration that oxytocin may regulate serotonin release and exert anxiolytic effects via direct activation of oxytocin receptor expressed in serotonergic neurons of the raphe nuclei. These results also have important implications for psychiatric disorders such as autism and depression in which both the oxytocin and serotonin systems have been implicated.
Figures

References
-
- Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG, Burbach JP. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology. 1995;136:4022–4028. - PubMed
-
- Aggleton JP. The amygdala: a functional analysis. Ed 2. London: Oxford UP; 2001.
-
- Bergquist F, Ludwig M. Dendritic transmitter release: a comparison of two model systems. J Neuroendocrinol. 2008;20:677–686. - PubMed
-
- Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. - PubMed
-
- Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
