Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90
- PMID: 19229106
- PMCID: PMC2648691
- DOI: 10.1172/JCI37613
Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90
Abstract
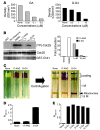
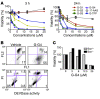
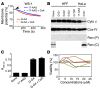
Although therapeutically targeting a single signaling pathway that drives tumor development and/or progression has been effective for a number of cancers, in many cases this approach has not been successful. Targeting networks of signaling pathways, instead of isolated pathways, may overcome this problem, which is probably due to the extreme heterogeneity of human tumors. However, the possibility that such networks may be spatially arranged in specialized subcellular compartments is not often considered in pathway-oriented drug discovery and may influence the design of new agents. Hsp90 is a chaperone protein that controls the folding of proteins in multiple signaling networks that drive tumor development and progression. Here, we report the synthesis and properties of Gamitrinibs, a class of small molecules designed to selectively target Hsp90 in human tumor mitochondria. Gamitrinibs were shown to accumulate in the mitochondria of human tumor cell lines and to inhibit Hsp90 activity by acting as ATPase antagonists. Unlike Hsp90 antagonists not targeted to mitochondria, Gamitrinibs exhibited a "mitochondriotoxic" mechanism of action, causing rapid tumor cell death and inhibiting the growth of xenografted human tumor cell lines in mice. Importantly, Gamitrinibs were not toxic to normal cells or tissues and did not affect Hsp90 homeostasis in cellular compartments other than mitochondria. Therefore, combinatorial drug design, whereby inhibitors of signaling networks are targeted to specific subcellular compartments, may generate effective anticancer drugs with novel mechanisms of action.
Figures




Comment in
-
Shock the heat shock network.J Clin Invest. 2009 Mar;119(3):445-8. doi: 10.1172/jci38681. J Clin Invest. 2009. PMID: 19306500 Free PMC article.
Similar articles
-
Preclinical characterization of mitochondria-targeted small molecule hsp90 inhibitors, gamitrinibs, in advanced prostate cancer.Clin Cancer Res. 2010 Oct 1;16(19):4779-88. doi: 10.1158/1078-0432.CCR-10-1818. Epub 2010 Sep 28. Clin Cancer Res. 2010. PMID: 20876793 Free PMC article.
-
Targeted inhibition of mitochondrial Hsp90 suppresses localised and metastatic prostate cancer growth in a genetic mouse model of disease.Br J Cancer. 2011 Feb 15;104(4):629-34. doi: 10.1038/bjc.2011.9. Epub 2011 Feb 1. Br J Cancer. 2011. PMID: 21285984 Free PMC article.
-
Shock the heat shock network.J Clin Invest. 2009 Mar;119(3):445-8. doi: 10.1172/jci38681. J Clin Invest. 2009. PMID: 19306500 Free PMC article.
-
Drug-mediated targeted disruption of multiple protein activities through functional inhibition of the Hsp90 chaperone complex.Curr Med Chem. 2007;14(29):3122-38. doi: 10.2174/092986707782793925. Curr Med Chem. 2007. PMID: 18220746 Review.
-
Targeting heat-shock-protein 90 (Hsp90) by natural products: geldanamycin, a show case in cancer therapy.Nat Prod Rep. 2013 Oct 11;30(10):1299-323. doi: 10.1039/c3np70012g. Epub 2013 Aug 12. Nat Prod Rep. 2013. PMID: 23934201 Review.
Cited by
-
New insights into TRAP1 pathway.Am J Cancer Res. 2012;2(2):235-48. Epub 2012 Feb 19. Am J Cancer Res. 2012. PMID: 22432061 Free PMC article.
-
mtUPR Modulation as a Therapeutic Target for Primary and Secondary Mitochondrial Diseases.Int J Mol Sci. 2023 Jan 12;24(2):1482. doi: 10.3390/ijms24021482. Int J Mol Sci. 2023. PMID: 36674998 Free PMC article. Review.
-
Targeting mitochondria for cancer therapy.Nat Rev Drug Discov. 2010 Jun;9(6):447-64. doi: 10.1038/nrd3137. Epub 2010 May 14. Nat Rev Drug Discov. 2010. PMID: 20467424 Review.
-
A Mitochondrial-targeted purine-based HSP90 antagonist for leukemia therapy.Oncotarget. 2017 Dec 11;8(68):112184-112198. doi: 10.18632/oncotarget.23097. eCollection 2017 Dec 22. Oncotarget. 2017. PMID: 29348817 Free PMC article.
-
Insight into nanoparticle cellular uptake and intracellular targeting.J Control Release. 2014 Sep 28;190:485-99. doi: 10.1016/j.jconrel.2014.06.038. Epub 2014 Jun 28. J Control Release. 2014. PMID: 24984011 Free PMC article. Review.

