PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV
- PMID: 19229109
- PMCID: PMC2648671
- DOI: 10.1172/JCI36604
PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV
Abstract
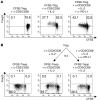
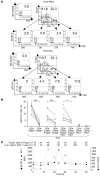
CD4+CD25+Foxp3+ Tregs suppress autoimmune responses. In addition, they limit T cell responses during chronic infection, thereby minimizing T cell-dependent immunopathology. We sought to investigate how Tregs are regulated in the livers of patients chronically infected with HCV, where they control the balance between an adequate protective immune response and suppression of immunopathology. We found that, despite accumulating and proliferating at sites of infection in the livers of patients chronically infected with HCV, Tregs were relatively less expanded than CD4+CD25+Foxp3- effector T cells. The relative lower expansion of intrahepatic Tregs coincided with their upregulation of programmed death-1 (PD-1). PD-1 expression inversely correlated with both Treg proliferation and clinical markers of immune suppression in vivo. Consistent with the possibility that PD-1 controls Tregs, blockade of the interaction between PD-1 and programmed death-1 ligand 1 (PD-L1) enhanced the in vitro expansion and function of Tregs isolated from the livers of patients chronically infected with HCV. Blockade of the interaction between PD-L1 and B7.1 also improved the proliferation of these cells. Interestingly, both PD-1 and phosphorylated STAT-5 were overexpressed in intrahepatic Tregs in a parallel fashion in steady disease conditions, and in an alternate-fluctuating fashion during the course of severe hepatitis reactivation. Notably, PD-L1 blockade upregulated STAT-5 phosphorylation in Tregs ex vivo. These data suggest that PD-L1 negatively regulates Tregs at sites of chronic inflammation by controlling STAT-5 phosphorylation.
Figures







Comment in
-
PD-1 tempers Tregs in chronic HCV infection.J Clin Invest. 2009 Mar;119(3):450-3. doi: 10.1172/jci38661. J Clin Invest. 2009. PMID: 19306502 Free PMC article.
References
-
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. - PubMed
-
- Baecher-Allan C., Brown J.A., Freeman G.J., Hafler D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245–1253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

