Structural basis for HIV-1 DNA integration in the human genome, role of the LEDGF/P75 cofactor
- PMID: 19229293
- PMCID: PMC2670869
- DOI: 10.1038/emboj.2009.41
Structural basis for HIV-1 DNA integration in the human genome, role of the LEDGF/P75 cofactor
Abstract
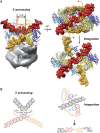
Integration of the human immunodeficiency virus (HIV-1) cDNA into the human genome is catalysed by integrase. Several studies have shown the importance of the interaction of cellular cofactors with integrase for viral integration and infectivity. In this study, we produced a stable and functional complex between the wild-type full-length integrase (IN) and the cellular cofactor LEDGF/p75 that shows enhanced in vitro integration activity compared with the integrase alone. Mass spectrometry analysis and the fitting of known atomic structures in cryo negatively stain electron microscopy (EM) maps revealed that the functional unit comprises two asymmetric integrase dimers and two LEDGF/p75 molecules. In the presence of DNA, EM revealed the DNA-binding sites and indicated that, in each asymmetric dimer, one integrase molecule performs the catalytic reaction, whereas the other one positions the viral DNA in the active site of the opposite dimer. The positions of the target and viral DNAs for the 3' processing and integration reaction shed light on the integration mechanism, a process with wide implications for the understanding of viral-induced pathologies.
Figures




Similar articles
-
Structural and functional role of INI1 and LEDGF in the HIV-1 preintegration complex.PLoS One. 2013 Apr 11;8(4):e60734. doi: 10.1371/journal.pone.0060734. Print 2013. PLoS One. 2013. PMID: 23593299 Free PMC article.
-
Virus evolution reveals an exclusive role for LEDGF/p75 in chromosomal tethering of HIV.PLoS Pathog. 2007 Mar;3(3):e47. doi: 10.1371/journal.ppat.0030047. PLoS Pathog. 2007. PMID: 17397262 Free PMC article.
-
Implication of serine residues 271, 273, and 275 in the human immunodeficiency virus type 1 cofactor activity of lens epithelium-derived growth factor/p75.J Virol. 2010 Jan;84(2):740-52. doi: 10.1128/JVI.01043-09. Epub 2009 Nov 4. J Virol. 2010. PMID: 19889764 Free PMC article.
-
The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy.Virology. 2013 Jan 5;435(1):102-9. doi: 10.1016/j.virol.2012.09.033. Virology. 2013. PMID: 23217620 Review.
-
Integrase, LEDGF/p75 and HIV replication.Cell Mol Life Sci. 2008 May;65(9):1403-24. doi: 10.1007/s00018-008-7540-5. Cell Mol Life Sci. 2008. PMID: 18264802 Free PMC article. Review.
Cited by
-
Nup153 Unlocks the Nuclear Pore Complex for HIV-1 Nuclear Translocation in Nondividing Cells.J Virol. 2018 Sep 12;92(19):e00648-18. doi: 10.1128/JVI.00648-18. Print 2018 Oct 1. J Virol. 2018. PMID: 29997211 Free PMC article.
-
Intasome architecture and chromatin density modulate retroviral integration into nucleosome.Retrovirology. 2015 Feb 7;12:13. doi: 10.1186/s12977-015-0145-9. Retrovirology. 2015. PMID: 25807893 Free PMC article.
-
A cooperative and specific DNA-binding mode of HIV-1 integrase depends on the nature of the metallic cofactor and involves the zinc-containing N-terminal domain.Nucleic Acids Res. 2010 Jun;38(11):3692-708. doi: 10.1093/nar/gkq087. Epub 2010 Feb 17. Nucleic Acids Res. 2010. PMID: 20164093 Free PMC article.
-
Changes in the accessibility of the HIV-1 Integrase C-terminus in the presence of cellular proteins.Retrovirology. 2010 Apr 5;7:27. doi: 10.1186/1742-4690-7-27. Retrovirology. 2010. PMID: 20367881 Free PMC article.
-
Repair of oxidative DNA base damage in the host genome influences the HIV integration site sequence preference.PLoS One. 2014 Jul 22;9(7):e103164. doi: 10.1371/journal.pone.0103164. eCollection 2014. PLoS One. 2014. PMID: 25051054 Free PMC article.
References
-
- Agapkina J, Smolov M, Barbe S, Zubin E, Zatsepin T, Deprez E, Le BM, Mouscadet JF, Gottikh M (2006) Probing of HIV-1 integrase/DNA interactions using novel analogs of viral DNA. J Biol Chem 281: 11530–11540 - PubMed
-
- Busschots K, Voet A, De MM, Rain JC, Emiliani S, Benarous R, Desender L, Debyser Z, Christ F (2007) Identification of the LEDGF/p75 binding site in HIV-1 integrase. J Mol Biol 365: 1480–1492 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

