The tail sheath structure of bacteriophage T4: a molecular machine for infecting bacteria
- PMID: 19229296
- PMCID: PMC2670864
- DOI: 10.1038/emboj.2009.36
The tail sheath structure of bacteriophage T4: a molecular machine for infecting bacteria
Erratum in
- EMBO J. 2012 Aug 15;31(16):3507
Abstract
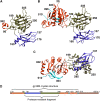
The contractile tail of bacteriophage T4 is a molecular machine that facilitates very high viral infection efficiency. Its major component is a tail sheath, which contracts during infection to less than half of its initial length. The sheath consists of 138 copies of the tail sheath protein, gene product (gp) 18, which surrounds the central non-contractile tail tube. The contraction of the sheath drives the tail tube through the outer membrane, creating a channel for the viral genome delivery. A crystal structure of about three quarters of gp18 has been determined and was fitted into cryo-electron microscopy reconstructions of the tail sheath before and after contraction. It was shown that during contraction, gp18 subunits slide over each other with no apparent change in their structure.
Figures





Comment in
-
How viruses infect bacteria?EMBO J. 2009 Apr 8;28(7):797-8. doi: 10.1038/emboj.2009.71. EMBO J. 2009. PMID: 19352408 Free PMC article. No abstract available.
References
-
- Ackermann H-W (2006) Classification of bacteriophages. In The Bacteriophages, Calendar R (ed), 2nd edn, pp 8–16. Oxford University Press: New York, NY
-
- Afonine PV, Grosse-Kunstleve RW, Adams PD (2005) The Phenix refinement framework. CCP4 Newsletter. Number 42, Contribution 8
-
- Arisaka F, Engel J, Horst K (1981) Contraction and dissociation of the bacteriophage T4 tail sheath induced by heat and urea. Prog Clin Biol Res 64: 365–379 - PubMed
-
- Arisaka F, Takeda S, Funane K, Nishijima N, Ishii S (1990) Structural studies of the contractile tail sheath protein of bacteriophage T4. 2. Structural analyses of the tail sheath protein, gp18, by limited proteolysis, immunoblotting, and immunoelectron microscopy. Biochemistry 29: 5057–5062 - PubMed
-
- Arisaka F, Tschopp J, van Driel R, Engel J (1979) Reassembly of the bacteriophage T4 tail from the core-baseplate and the monomeric sheath protein P18: a co-operative association process. J Mol Biol 132: 369–386 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

