Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP
- PMID: 19229313
- PMCID: PMC2636892
- DOI: 10.1371/journal.ppat.1000303
Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP
Abstract
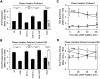
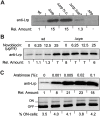
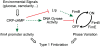
Type 1 fimbriae are a crucial factor for the virulence of uropathogenic Escherichia coli during the first steps of infection by mediating adhesion to epithelial cells. They are also required for the consequent colonization of the tissues and for invasion of the uroepithelium. Here, we studied the role of the specialized signal transduction system CRP-cAMP in the regulation of type 1 fimbriation. Although initially discovered by regulating carbohydrate metabolism, the CRP-cAMP complex controls a major regulatory network in Gram-negative bacteria, including a broad subset of genes spread into different functional categories of the cell. Our results indicate that CRP-cAMP plays a dual role in type 1 fimbriation, affecting both the phase variation process and fimA promoter activity, with an overall repressive outcome on fimbriation. The dissection of the regulatory pathway let us conclude that CRP-cAMP negatively affects FimB-mediated recombination by an indirect mechanism that requires DNA gyrase activity. Moreover, the underlying studies revealed that CRP-cAMP controls the expression of another global regulator in Gram-negative bacteria, the leucine-responsive protein Lrp. CRP-cAMP-mediated repression is limiting the switch from the non-fimbriated to the fimbriated state. Consistently, a drop in the intracellular concentration of cAMP due to altered physiological conditions (e.g. growth in presence of glucose) increases the percentage of fimbriated cells in the bacterial population. We also provide evidence that the repression of type 1 fimbriae by CRP-cAMP occurs during fast growth conditions (logarithmic phase) and is alleviated during slow growth (stationary phase), which is consistent with an involvement of type 1 fimbriae in the adaptation to stress conditions by promoting biofilm growth or entry into host cells. Our work suggests that the metabolic sensor CRP-cAMP plays a role in coupling the expression of type 1 fimbriae to environmental conditions, thereby also affecting subsequent attachment and colonization of host tissues.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
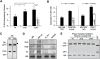
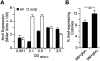
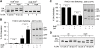

References
-
- Ullmann A, Monod J. Cyclic AMP as an antagonist of catabolite repression in Escherichia coli. FEBS Lett. 1968;2:57–60. - PubMed
-
- Kolb A, Busby S, Buc II, Garges S, Adhya S. Transcriptional Regulation by cAMP and its Receptor Protein. Ann Rev Biochem. 1993;62:749–797. - PubMed
-
- Lory S, Wolfgang M, Lee V, Smith R. The multi-talented bacterial adenylate cyclases. Int J Med Microbiol. 2004;293:479–482. - PubMed
-
- Baker DA, Kelly JM. Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol Microbiol. 2004;52:1229–1242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

