Poly (ADP-ribose) polymerase 1 is required for protein localization to Cajal body
- PMID: 19229318
- PMCID: PMC2637609
- DOI: 10.1371/journal.pgen.1000387
Poly (ADP-ribose) polymerase 1 is required for protein localization to Cajal body
Abstract
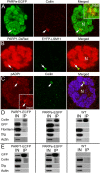
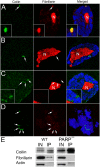
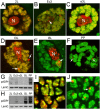
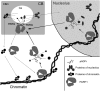
Recently, the nuclear protein known as Poly (ADP-ribose) Polymerase1 (PARP1) was shown to play a key role in regulating transcription of a number of genes and controlling the nuclear sub-organelle nucleolus. PARP1 enzyme is known to catalyze the transfer of ADP-ribose to a variety of nuclear proteins. At present, however, while we do know that the main acceptor for pADPr in vivo is PARP1 protein itself, by PARP1 automodification, the significance of PARP1 automodification for in vivo processes is not clear. Therefore, we investigated the roles of PARP1 auto ADP-ribosylation in dynamic nuclear processes during development. Specifically, we discovered that PARP1 automodification is required for shuttling key proteins into Cajal body (CB) by protein non-covalent interaction with pADPr in vivo. We hypothesize that PARP1 protein shuttling follows a chain of events whereby, first, most unmodified PARP1 protein molecules bind to chromatin and accumulate in nucleoli, but then, second, upon automodification with poly(ADP-ribose), PARP1 interacts non-covalently with a number of nuclear proteins such that the resulting protein-pADPr complex dissociates from chromatin into CB.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Poly(ADP-Ribose) polymerase 1 (PARP-1) regulates ribosomal biogenesis in Drosophila nucleoli.PLoS Genet. 2012 Jan;8(1):e1002442. doi: 10.1371/journal.pgen.1002442. Epub 2012 Jan 5. PLoS Genet. 2012. PMID: 22242017 Free PMC article.
-
Using Drosophila Genetics to Identify Factors that Affect PARP1 Activity In Vivo.Methods Mol Biol. 2023;2609:339-352. doi: 10.1007/978-1-0716-2891-1_20. Methods Mol Biol. 2023. PMID: 36515845
-
Nucleosomal core histones mediate dynamic regulation of poly(ADP-ribose) polymerase 1 protein binding to chromatin and induction of its enzymatic activity.J Biol Chem. 2007 Nov 2;282(44):32511-9. doi: 10.1074/jbc.M705989200. Epub 2007 Sep 7. J Biol Chem. 2007. PMID: 17827147
-
Poly-ADP-ribose polymerase: machinery for nuclear processes.Mol Aspects Med. 2013 Dec;34(6):1124-37. doi: 10.1016/j.mam.2013.04.001. Epub 2013 Apr 25. Mol Aspects Med. 2013. PMID: 23624145 Free PMC article. Review.
-
Reprogramming cellular events by poly(ADP-ribose)-binding proteins.Mol Aspects Med. 2013 Dec;34(6):1066-87. doi: 10.1016/j.mam.2012.12.005. Epub 2012 Dec 23. Mol Aspects Med. 2013. PMID: 23268355 Free PMC article. Review.
Cited by
-
Poly(ADP-ribosyl)ating enzymes coordinate changes in the expression of metabolic genes with developmental progression.Sci Rep. 2023 Nov 20;13(1):20320. doi: 10.1038/s41598-023-47691-8. Sci Rep. 2023. PMID: 37985852 Free PMC article.
-
RNA Regulation by Poly(ADP-Ribose) Polymerases.Mol Cell. 2015 Jun 18;58(6):959-69. doi: 10.1016/j.molcel.2015.01.037. Mol Cell. 2015. PMID: 26091344 Free PMC article. Review.
-
Poly(ADP-ribosyl)ating enzymes cooperate to coordinate development.Sci Rep. 2022 Dec 21;12(1):22120. doi: 10.1038/s41598-022-26530-2. Sci Rep. 2022. PMID: 36543866 Free PMC article.
-
Poly(ADP-ribosyl)ation of heterogeneous nuclear ribonucleoproteins modulates splicing.Nucleic Acids Res. 2009 Jun;37(11):3501-13. doi: 10.1093/nar/gkp218. Epub 2009 Apr 3. Nucleic Acids Res. 2009. PMID: 19346337 Free PMC article.
-
PARG Protein Regulation Roles in Drosophila Longevity Control.Int J Mol Sci. 2024 Jun 4;25(11):6189. doi: 10.3390/ijms25116189. Int J Mol Sci. 2024. PMID: 38892377 Free PMC article.
References
-
- De Murcia G, Shall S. From DNA damage and stress signalling to cell death Poly (ADP-Ribosylation) Reactions. New York: Oxford University Press; 2000.
-
- Meder VS, Boeglin M, de Murcia G, Schreiber V. PARP-1 and PARP-2 interact with nucleophosmin/B23 and accumulate in transcriptionally active nucleoli. J Cell Sci. 2005;118:211–222. - PubMed
-
- Pinnola A, Naumova N, Shah M, Tulin AV. Nucleosomal core histones mediate dynamic regulation of PARP1 protein binding to chromatin and induction of PARP1 enzymatic activity. J Biol Chem. 2007;282:32511–32519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

