Beyond Reppe: building substituted arenes by [2+2+2] cycloadditions of alkynes
- PMID: 19229917
- PMCID: PMC2898745
- DOI: 10.1002/anie.200804651
Beyond Reppe: building substituted arenes by [2+2+2] cycloadditions of alkynes
Abstract
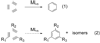
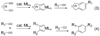
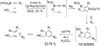
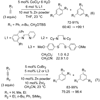
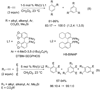
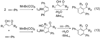
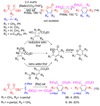
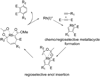
Synthetic sequel: The transition-metal-catalyzed [2+2+2] cycloaddition is an established method for the construction of carbocyclic frameworks but is often plagued by poor selectivity. Recent literature paints a promising picture--a more general metal-catalyzed [2+2+2] cycloaddition can be accomplished intermolecularly using three separate alkynes to furnish highly substituted arenas (see scheme).
Figures




References
-
-
For recent reviews on the synthesis of ring systems using [2+2+2] cycloadditions see: Kotha S, Brahmachary E, Lahiri K. Eur. J. Org. Chem. 2005:4741–4767. Saito S, Yamamoto Y. Chem. Rev. 2000;100:2901–2915. Chopade PR, Louie J. Adv. Synth. Catal. 2006;348:2307–2327. Tanaka K. Synlett. 2007;10:1977–1993. Shibata T, Tsuchikama K. Org. Biomol. Chem. 2008;6:1317–1323. Yamamoto Y. Curr. Org. Chem. 2005;9:503–1519. Heller B, Hapke M. Chem Soc. Rev. 2007;36:1085–1094. Varela JA, Saá C. Chem. Rev. 2003;103:3787–3801. Henry GD. Tetrahedron. 2004;60:6043–6061. Agenet N, Busine O, Slowinski F, Gandon V, Aubert C, Malacria M. Org. React. 2007;68:1–302.
-
-
- Reppe W, Schweckendiek WJ. Liebigs Ann. Chem. 1948;560:104–116.
-
- Takahashi T, Xi Z, Yamazaki A, Liu Y, Nakajima K, Kotora M. J. Am. Chem. Soc. 1998;120:1672–1680. and references therein.
-
- Vollhardt PC. Angew. Chem. Int. Ed. Engl. 1984;23:539–556.
- Schore NEK. Chem. Rev. 1988;88:1081–1119.
-
-
Suzuki D, Urabe H, Sato F. J. Am. Chem. Soc. 2001;123:7925–7926. For the synthesis of substituted pyridines from nitriles see: b) Suzuki D, Nobe Y, Watai Y, Tanaka R, Takayama Y, Sato F, Urabe H. J. Am. Chem. Soc. 2005;127:7474–7479. Tanaka R, Yuza A, Watai Y, Suzuki D, Takayama Y, Sato F, Urabe H. J. Am. Chem. Soc. 2005;127:7774–7780.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

