Bile acids: chemistry, physiology, and pathophysiology
- PMID: 19230041
- PMCID: PMC2653380
- DOI: 10.3748/wjg.15.804
Bile acids: chemistry, physiology, and pathophysiology
Abstract
The family of bile acids includes a group of molecular species of acidic steroids with very peculiar physical-chemical and biological characteristics. They are synthesized by the liver from cholesterol through several complementary pathways that are controlled by mechanisms involving fine-tuning by the levels of certain bile acid species. Although their best-known role is their participation in the digestion and absorption of fat, they also play an important role in several other physiological processes. Thus, genetic abnormalities accounting for alterations in their synthesis, biotransformation and/or transport may result in severe alterations, even leading to lethal situations for which the sole therapeutic option may be liver transplantation. Moreover, the increased levels of bile acids reached during cholestatic liver diseases are known to induce oxidative stress and apoptosis, resulting in damage to the liver parenchyma and, eventually, extrahepatic tissues. When this occurs during pregnancy, the outcome of gestation may be challenged. In contrast, the physical-chemical and biological properties of these compounds have been used as the bases for the development of drugs and as pharmaceutical tools for the delivery of active agents.
Figures

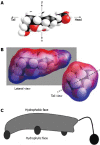

References
-
- Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. - PubMed
-
- Babu P, Sangeetha NM, Maitra U. Supramolecular chemistry of bile acid derivatives: formation of gels. Macromol Symp. 2006;241:60–67.
-
- Hofmann AF, Mysels KJ. Bile salts as biological surfactants. Colloids Surfaces. 1988;30:145–173.
-
- Hofmann AF, Sjövall J, Kurz G, Radominska A, Schteingart CD, Tint GS, Vlahcevic ZR, Setchell KD. A proposed nomenclature for bile acids. J Lipid Res. 1992;33:599–604. - PubMed
-
- Warren DB, Chalmers DK, Hutchison K, Dang W, Pouton CW. Molecular dynamics simulations of spontaneous bile salt aggregation. Colloids Surfaces A. 2006;280:182–193.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

