Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study
- PMID: 19230772
- PMCID: PMC4086808
- DOI: 10.1016/S1470-2045(09)70003-8
Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study
Abstract
Background: Drug treatments for patients with high-risk myelodysplastic syndromes provide no survival advantage. In this trial, we aimed to assess the effect of azacitidine on overall survival compared with the three commonest conventional care regimens.
Methods: In a phase III, international, multicentre, controlled, parallel-group, open-label trial, patients with higher-risk myelodysplastic syndromes were randomly assigned one-to-one to receive azacitidine (75 mg/m(2) per day for 7 days every 28 days) or conventional care (best supportive care, low-dose cytarabine, or intensive chemotherapy as selected by investigators before randomisation). Patients were stratified by French-American-British and international prognostic scoring system classifications; randomisation was done with a block size of four. The primary endpoint was overall survival. Efficacy analyses were by intention to treat for all patients assigned to receive treatment. This study is registered with ClinicalTrials.gov, number NCT00071799.
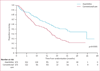
Findings: Between Feb 13, 2004, and Aug 7, 2006, 358 patients were randomly assigned to receive azacitidine (n=179) or conventional care regimens (n=179). Four patients in the azacitidine and 14 in the conventional care groups received no study drugs but were included in the intention-to-treat efficacy analysis. After a median follow-up of 21.1 months (IQR 15.1-26.9), median overall survival was 24.5 months (9.9-not reached) for the azacitidine group versus 15.0 months (5.6-24.1) for the conventional care group (hazard ratio 0.58; 95% CI 0.43-0.77; stratified log-rank p=0.0001). At last follow-up, 82 patients in the azacitidine group had died compared with 113 in the conventional care group. At 2 years, on the basis of Kaplan-Meier estimates, 50.8% (95% CI 42.1-58.8) of patients in the azacitidine group were alive compared with 26.2% (18.7-34.3) in the conventional care group (p<0.0001). Peripheral cytopenias were the most common grade 3-4 adverse events for all treatments.
Interpretation: Treatment with azacitidine increases overall survival in patients with higher-risk myelodysplastic syndromes relative to conventional care.
Conflict of interest statement
PF has participated in advisory board meetings for Celgene, Roche, Amgen, GlaxoSmithKlein, Merck, Novartis, Johnson and Johnson, and Cephalon. GJM has served as an advisory board member and consultant for Celgene, Amgen, and Genzyme, and as an advisory board member for Pharmion (now Celgene) and Johnson and Johnson. EH-L has participated in advisory board meetings for Celgene and Amgen and has given paid testimony for Celgene. VS has received honoraria from Celgene, Novartis, and Johnson and Johnson for lecturing. CF has no conflicts of interest. AG is a consultant to Celgene and participates on their speakers’ bureau. RS has nothing to disclose. NG has received honoraria for lecturing for Novartis, Roche, Celgene, and Janssen-Cilag and research support from Novartis, and Celgene. GS is a consultant for Celgene. AL has received honoraria from Celgene. SDG is a consultant for and owns stock in Celgene. JFS has participated on an advisory board for and has received honoraria from Celgene; he was a member of an advisory board and speaker’s bureau for Pharmion (now Celgene) and he received honoraria from them. JMB is a consultant for Celgene, Novartis, and Johnson and Johnson and is a member of a speakers’ bureau for Celgene. JBy is a consultant for Celgene. JBa, LZ, DM, and CLB are employees of Celgene and own stock in the company. LRS has received research funding and honoraria from Celgene.
Figures


Comment in
-
Improving survival in myelodysplastic syndromes.Lancet Oncol. 2009 Mar;10(3):200-1. doi: 10.1016/S1470-2045(09)70048-8. Lancet Oncol. 2009. PMID: 19261248 No abstract available.
References
-
- Sloand EM. Myelodysplastic syndromes: introduction. Semin Hematol. 2008;45:1–2. - PubMed
-
- Valent P, Horny HP, Bennett JM, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: consensus statements and report from a working conference. Leuk Res. 2007;31:72–36. - PubMed
-
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. - PubMed
-
- Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. - PubMed
-
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2098. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

