Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn's disease
- PMID: 19230854
- PMCID: PMC6326776
- DOI: 10.1053/j.gastro.2008.12.046
Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn's disease
Abstract
Background & aims: Genetic variations that affect innate immunity increase risk of ileal Crohn's disease (CD). However, the penetrance of susceptibility genes, including NOD2, is low, suggesting additional risk factors. Neutralizing autoantibodies (Ab) against granulocyte-macrophage colony-stimulating factor (GM-CSF Ab) reduce neutrophil antimicrobial function in patients with primary alveolar proteinosis (PAP). We investigated whether GM-CSF Ab regulates neutrophil function in CD.
Methods: Serum samples from 354 adult and pediatric patients with inflammatory bowel disease (IBD) were analyzed for GM-CSF Ab and IBD markers. Levels of GM-CSF Ab were compared with patients' CD features and neutrophil function. Intestinal barrier function and nonsteroidal anti-inflammatory drug (NSAID)-induced injury were assessed in GM-CSF-null and NOD2-null mice.
Results: Median GM-CSF Ab levels increased from 0.4 microg/mL in control serum to 2.4 microg/mL in pediatric CD and 11.7 microg/mL in adult CD serum and were associated with ileal involvement (P<.001). Ileal location, duration of disease, and increased GM-CSF Ab levels were associated with stricturing/penetrating behavior (odds ratio, 2.2; P=.018). The positive and negative predictive values of GM-CSF Ab for stricturing/penetrating behavior were comparable with that of other IBD serum markers. CD patients with increased GM-CSF Ab had reduced neutrophil phagocytic capacity and increased accumulation of pSTAT3+ neutrophils in the affected ileum. GM-CSF-null mice and NOD2-null mice in which GM-CSF was neutralized had defects in mucosal barrier function and developed a transmural ileitis following NSAID exposure.
Conclusions: GM-CSF regulates ileal homeostasis in CD and in mouse models. CD patients with increases in serum GM-CSF Ab might benefit from GM-CSF administration.
Figures



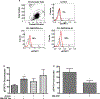

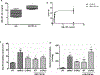

Similar articles
-
Granulocyte-macrophage colony stimulating factor blockade promotes ccr9(+) lymphocyte expansion in Nod2 deficient mice.Inflamm Bowel Dis. 2011 Dec;17(12):2443-55. doi: 10.1002/ibd.21672. Epub 2011 Mar 4. Inflamm Bowel Dis. 2011. PMID: 21381154 Free PMC article.
-
Innate dysfunction promotes linear growth failure in pediatric Crohn's disease and growth hormone resistance in murine ileitis.Inflamm Bowel Dis. 2012 Feb;18(2):236-45. doi: 10.1002/ibd.21689. Epub 2011 Feb 18. Inflamm Bowel Dis. 2012. PMID: 21337672 Free PMC article.
-
Granulocyte-macrophage colony-stimulating factor autoantibodies: a marker of aggressive Crohn's disease.Inflamm Bowel Dis. 2013 Jul;19(8):1671-80. doi: 10.1097/MIB.0b013e318281f506. Inflamm Bowel Dis. 2013. PMID: 23749272 Free PMC article.
-
Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn's disease.Am J Physiol Gastrointest Liver Physiol. 2014 Mar;306(6):G455-65. doi: 10.1152/ajpgi.00409.2013. Epub 2014 Feb 6. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24503766 Review.
-
Pulmonary alveolar proteinosis: a bench-to-bedside story of granulocyte-macrophage colony-stimulating factor dysfunction.Chest. 2009 Aug;136(2):571-577. doi: 10.1378/chest.08-2943. Chest. 2009. PMID: 19666756 Review.
Cited by
-
Enterocyte STAT5 promotes mucosal wound healing via suppression of myosin light chain kinase-mediated loss of barrier function and inflammation.EMBO Mol Med. 2012 Feb;4(2):109-24. doi: 10.1002/emmm.201100192. Epub 2012 Jan 9. EMBO Mol Med. 2012. PMID: 22228679 Free PMC article.
-
Association Between Plasma Level of Collagen Type III Alpha 1 Chain and Development of Strictures in Pediatric Patients With Crohn's Disease.Clin Gastroenterol Hepatol. 2019 Aug;17(9):1799-1806. doi: 10.1016/j.cgh.2018.09.008. Epub 2018 Sep 10. Clin Gastroenterol Hepatol. 2019. PMID: 30213581 Free PMC article.
-
Butanol Purified Food Allergy Herbal Formula-2 Has an Immunomodulating Effect ex-vivo in Pediatric Crohn's Disease Subjects.Front Med (Lausanne). 2021 Nov 29;8:782859. doi: 10.3389/fmed.2021.782859. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34926527 Free PMC article.
-
Secondary causes of inflammatory bowel diseases.World J Gastroenterol. 2020 Jul 28;26(28):3998-4017. doi: 10.3748/wjg.v26.i28.3998. World J Gastroenterol. 2020. PMID: 32821067 Free PMC article. Review.
-
Pattern Recognition Receptor Signaling and Cytokine Networks in Microbial Defenses and Regulation of Intestinal Barriers: Implications for Inflammatory Bowel Disease.Gastroenterology. 2022 May;162(6):1602-1616.e6. doi: 10.1053/j.gastro.2021.12.288. Epub 2022 Feb 9. Gastroenterology. 2022. PMID: 35149024 Free PMC article. Review.
References
-
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002;347:911–20. - PubMed
-
- Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol 2007;179:3638–47. - PubMed
-
- Rosenbloom AJ, Linden PK, Dorrance A, Penkosky N, Cohen-Melamed MH, Pinsky MR. Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest 2005;127:2139–50. - PubMed
-
- Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, Puchalski JT, Hauck DM, Trapnell BC. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med 2007;356:567–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

