Continuous-flow enzyme assay on a microfluidic chip for monitoring glycerol secretion from cultured adipocytes
- PMID: 19231843
- PMCID: PMC2659456
- DOI: 10.1021/ac8026965
Continuous-flow enzyme assay on a microfluidic chip for monitoring glycerol secretion from cultured adipocytes
Abstract
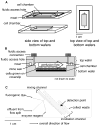
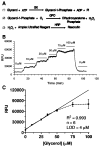
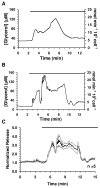
A dual-chip microfluidic platform that coupled perfusion of cultured adipocytes with on-line fluorescence-based enzyme assay was developed to monitor glycerol secretions in real time from cultured adipocytes. The perfusion cell chip, which could be reversibly sealed to allow reloading of cells and reuse of the chip, was shown by modeling to generate low shear stress on the cells under study. Effluent from the perfusion chip was pumped into an enzyme assay chip for monitoring of secretion from the cells. The on-line enzyme assay had a limit of detection (LOD) of 4 microM glycerol. The temporal resolution of the combined system for detecting changes in glycerol concentration was 90 s. The microfluidic device was used to continuously monitor glycerol secretion from murine 3T3-L1 adipocytes, grown and differentiated on glass coverslips, for at least 2 h. An average basal glycerol concentration of 28 microM was detected in the effluent. Pharmacological treatment with a beta-adrenergic agonist to stimulate lipolysis evoked a 3-fold increase in glycerol secretion followed by sustained release that was 40% higher than basal concentration. The ability to monitor changes in cellular secretion over time may provide insight into adipocyte metabolism and the dysregulation that occurs with obesity-related disorders.
Figures

References
-
- Davidsson R, Boketoft A, Bristulf J, Kotarsky K, Olde B, Owman C, Bengtsson M, Laurell T, Emneus J. Anal Chem. 2004;76:4715–4720. - PubMed
-
- Cheng W, Klauke N, Sedgwick H, Smith GL, Cooper JM. Lab Chip. 2006;6:1424– 1431. - PubMed
-
- Lau AY, Hung PJ, Wu AR, Lee LP. Lab Chip. 2006;6:1510–1515. - PubMed
-
- El-Ali J, Sorger PK, Jensen KF. Nature. 2006;442:403–411. - PubMed
-
- Kim L, Toh YC, Voldman J, Yu H. Lab Chip. 2007;7:681–694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

