Accelerated photobleaching of a cyanine dye in the presence of a ternary target DNA, PNA probe, dye catalytic complex: a molecular diagnostic
- PMID: 19231844
- PMCID: PMC2827246
- DOI: 10.1021/ac702519k
Accelerated photobleaching of a cyanine dye in the presence of a ternary target DNA, PNA probe, dye catalytic complex: a molecular diagnostic
Abstract
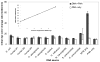
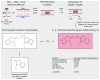
In many settings, molecular testing is needed but unavailable due to complexity and cost. Simple, rapid, and specific DNA detection technologies would provide important alternatives to existing detection methods. Here we report a novel, rapid nucleic acid detection method based on the accelerated photobleaching of the light-sensitive cyanine dye, 3,3'-diethylthiacarbocyanine iodide (DiSC(2)(3) I(-)), in the presence of a target genomic DNA and a complementary peptide nucleic acid (PNA) probe. On the basis of the UV-vis, circular dichroism, and fluorescence spectra of DiSC(2)(3) with PNA-DNA oligomer duplexes and on characterization of a product of photolysis of DiSC(2)(3) I(-), a possible reaction mechanism is proposed. We propose that (1) a novel complex forms between dye, PNA, and DNA, (2) this complex functions as a photosensitizer producing (1)O(2), and (3) the (1)O(2) produced promotes photobleaching of dye molecules in the mixture. Similar cyanine dyes (DiSC(3)(3), DiSC(4)(3), DiSC(5)(3), and DiSC(py)(3)) interact with preformed PNA-DNA oligomer duplexes but do not demonstrate an equivalent accelerated photobleaching effect in the presence of PNA and target genomic DNA. The feasibility of developing molecular diagnostic assays based on the accelerated photobleaching (the smartDNA assay) that results from the novel complex formed between DiSC(2)(3) and PNA-DNA is under way.
Figures


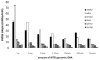

Similar articles
-
Effect of PNA backbone modifications on cyanine dye binding to PNA-DNA duplexes investigated by optical spectroscopy and molecular dynamics simulations.J Am Chem Soc. 2005 Mar 16;127(10):3339-45. doi: 10.1021/ja045145a. J Am Chem Soc. 2005. PMID: 15755150
-
Unconventional method based on circular dichroism to detect peanut DNA in food by means of a PNA probe and a cyanine dye.Chirality. 2005 Nov;17(9):515-21. doi: 10.1002/chir.20194. Chirality. 2005. PMID: 16170795
-
A PNA-DNA2 Triple-Helix Molecular Switch-Based Colorimetric Sensor for Sensitive and Specific Detection of microRNAs from Cancer Cells.Chembiochem. 2020 Sep 14;21(18):2667-2675. doi: 10.1002/cbic.202000155. Epub 2020 May 28. Chembiochem. 2020. PMID: 32304168
-
Fast and easy colorimetric tests for single mismatch recognition by PNA-DNA duplexes with the diethylthiadicarbocyanine dye and succinyl-beta-cyclodextrin.J Biochem Biophys Methods. 2007 Aug 1;70(5):735-41. doi: 10.1016/j.jbbm.2007.04.003. Epub 2007 Apr 14. J Biochem Biophys Methods. 2007. PMID: 17524491
-
FIT probes: peptide nucleic acid probes with a fluorescent base surrogate enable real-time DNA quantification and single nucleotide polymorphism discovery.Anal Biochem. 2008 Apr 15;375(2):318-30. doi: 10.1016/j.ab.2008.01.009. Epub 2008 Jan 12. Anal Biochem. 2008. PMID: 18249184
Cited by
-
Molecular beacons of xeno-nucleic acid for detecting nucleic acid.Theranostics. 2013 May 5;3(6):395-408. doi: 10.7150/thno.5935. Print 2013. Theranostics. 2013. PMID: 23781286 Free PMC article. Review.
-
Peptide nucleic acids: Advanced tools for biomedical applications.J Biotechnol. 2017 Oct 10;259:148-159. doi: 10.1016/j.jbiotec.2017.07.026. Epub 2017 Jul 29. J Biotechnol. 2017. PMID: 28764969 Free PMC article. Review.
-
Efficiency of a Tetracycline-Adjuvant Combination Against Multidrug Resistant Pseudomonas aeruginosa Tunisian Clinical Isolates.Antibiotics (Basel). 2020 Dec 17;9(12):919. doi: 10.3390/antibiotics9120919. Antibiotics (Basel). 2020. PMID: 33348867 Free PMC article.
References
-
- Nielsen PE, Egholm M, Berg RH, Buchardt O. Science. 1991;254(5037):1497–1500. - PubMed
-
- Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE. Nature. 1993;365(6446):566–8. - PubMed
-
- Kuhn H, Demidov VV, Coull JM, Fiandaca MJ, Gildea BD, Frank-Kamenetskii MD. J Am Chem Soc. 2002;124(6):1097–103. - PubMed
-
- Jensen KK, Orum H, Nielsen PE, Norden B. Biochemistry. 1997;36(16):5072–7. - PubMed
-
- Tomac S, Sarkar M, Ratilainen T, Wittung P, Nielsen PE, Norden B, Gralslund A. J Am Chem Soc. 1996;118:5544–52.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

