Multidrug resistance in bacteria
- PMID: 19231985
- PMCID: PMC2839888
- DOI: 10.1146/annurev.biochem.78.082907.145923
Multidrug resistance in bacteria
Abstract
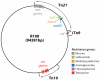
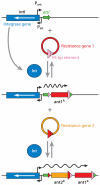
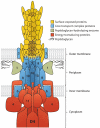
Large amounts of antibiotics used for human therapy, as well as for farm animals and even for fish in aquaculture, resulted in the selection of pathogenic bacteria resistant to multiple drugs. Multidrug resistance in bacteria may be generated by one of two mechanisms. First, these bacteria may accumulate multiple genes, each coding for resistance to a single drug, within a single cell. This accumulation occurs typically on resistance (R) plasmids. Second, multidrug resistance may also occur by the increased expression of genes that code for multidrug efflux pumps, extruding a wide range of drugs. This review discusses our current knowledge on the molecular mechanisms involved in both types of resistance.
Figures

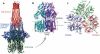


References
-
- Livermore DM. The need for new antibiotics. Clin. Microbiol. Infect. 2004;10(Suppl 4):1–9. - PubMed
-
- Hooper DC. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 2000;31(Suppl 2):S24–28. - PubMed
-
- Poehlsgaard J, Douthwaite S. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 2005;3:870–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

