A non-sequence-specific DNA binding mode of RAG1 is inhibited by RAG2
- PMID: 19232525
- PMCID: PMC2659343
- DOI: 10.1016/j.jmb.2009.02.020
A non-sequence-specific DNA binding mode of RAG1 is inhibited by RAG2
Abstract
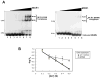
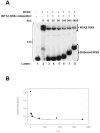
RAG1 and RAG2 proteins catalyze site-specific DNA cleavage reactions in V(D)J recombination, a process that assembles antigen receptor genes from component gene segments during lymphocyte development. The first step towards the DNA cleavage reaction is the sequence-specific association of the RAG proteins with the conserved recombination signal sequence (RSS), which flanks each gene segment in the antigen receptor loci. Questions remain as to the contribution of each RAG protein to recognition of the RSS. For example, while RAG1 alone is capable of recognizing the conserved elements of the RSS, it is not clear if or how RAG2 may enhance sequence-specific associations with the RSS. To shed light on this issue, we examined the association of RAG1, with and without RAG2, with consensus RSS versus non-RSS substrates using fluorescence anisotropy and gel mobility shift assays. The results indicate that while RAG1 can recognize the RSS, the sequence-specific interaction under physiological conditions is masked by a high-affinity non-sequence-specific DNA binding mode. Significantly, addition of RAG2 effectively suppressed the association of RAG1 with non-sequence-specific DNA, resulting in a large differential in binding affinity for the RSS versus the non-RSS sites. We conclude that this represents a major means by which RAG2 contributes to the initial recognition of the RSS and that, therefore, association of RAG1 with RAG2 is required for effective interactions with the RSS in developing lymphocytes.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

