A cellular model of Alzheimer's disease therapeutic efficacy: PKC activation reverses Abeta-induced biomarker abnormality on cultured fibroblasts
- PMID: 19233276
- PMCID: PMC2683973
- DOI: 10.1016/j.nbd.2009.02.003
A cellular model of Alzheimer's disease therapeutic efficacy: PKC activation reverses Abeta-induced biomarker abnormality on cultured fibroblasts
Abstract
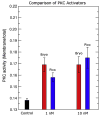
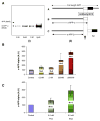
PKC signaling is critical for the non-toxic degradation of amyloid precursor protein (APP) and inhibition of GSK3beta, which controls phosphorylation of tau protein in Alzheimer's disease (AD). Thus the misregulation of PKC signaling could contribute to the origins of AD. Bryostatin, a potent PKC modulator, has the potential to ameliorate both the neurodegeneration and the recent memory loss associated with AD. As reported herein bryostatin and a potent synthetic analog (picolog) are found to cause stimulation of non-amyloidogenic pathways by increasing alpha-secretase activity and thus lowering the amount of toxic Abeta produced. Both bryostatin and picolog increased the secretion of the alpha-secretase product (s-APP-alpha) of APP at sub-nanomolar to nanomolar concentrations. A peripheral AD-Biomarker has previously been autopsy-validated. This Biomarker, based on bradykinin-induced differential phosphorylation of Erk1 and Erk2, has been used here to test the therapeutic efficacy both for bryostatin and picolog. Both of these PKC activators are then shown to convert the AD Erk1/2 phenotype of fibroblasts into the phenotype of "normal" control skin fibroblasts. This conversion occurred for both the abnormal Erk1/2 phenotype induced by application of Abeta(1-42) to the fibroblasts or the phenotype observed for fibroblasts of AD patients. The Abeta(1-42)-induction, and PKC modulator reversal of the AD Erk1/2 biomarker phenotype demonstrate the AD-Biomarker's potential to monitor both disease progression and treatment response. Additionally, this first demonstration of the therapeutic potential in AD of a synthetically accessible bryostatin analog warrants further preclinical advancement.
Figures



Similar articles
-
Bryostatin-1 vs. TPPB: dose-dependent APP processing and PKC-α, -δ, and -ε isoform activation in SH-SY5Y neuronal cells.J Mol Neurosci. 2012 Sep;48(1):234-44. doi: 10.1007/s12031-012-9816-3. Epub 2012 Jun 15. J Mol Neurosci. 2012. PMID: 22700373 Free PMC article.
-
Huperzine A regulates amyloid precursor protein processing via protein kinase C and mitogen-activated protein kinase pathways in neuroblastoma SK-N-SH cells over-expressing wild type human amyloid precursor protein 695.Neuroscience. 2007 Dec 5;150(2):386-95. doi: 10.1016/j.neuroscience.2007.09.022. Epub 2007 Sep 14. Neuroscience. 2007. PMID: 17945434
-
Nanoparticle-Encapsulated Bryostatin-1 Activates α-Secretase and PKC Isoforms In vitro and Facilitates Acquisition and Retention of Spatial Learning in an Alzheimer's Disease Mouse Model.Curr Alzheimer Res. 2020;17(14):1302-1310. doi: 10.2174/1567205018666210218155835. Curr Alzheimer Res. 2020. PMID: 33602091
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Preclinical and Clinical Studies on Bryostatins, A Class of Marine-Derived Protein Kinase C Modulators: A Mini-Review.Curr Top Med Chem. 2020;20(12):1124-1135. doi: 10.2174/1568026620666200325110444. Curr Top Med Chem. 2020. PMID: 32209043 Review.
Cited by
-
Conformation-activity relationships of polyketide natural products.Nat Prod Rep. 2015 Aug;32(8):1183-206. doi: 10.1039/c5np00014a. Nat Prod Rep. 2015. PMID: 25974024 Free PMC article. Review.
-
Design, synthesis, and evaluation of potent bryostatin analogs that modulate PKC translocation selectivity.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6721-6. doi: 10.1073/pnas.1015270108. Epub 2011 Mar 17. Proc Natl Acad Sci U S A. 2011. PMID: 21415363 Free PMC article.
-
Function through bio-inspired, synthesis-informed design: step-economical syntheses of designed kinase inhibitors†Dedicated to Max Malacria, a friend and scholar whose science and creative contributions to step-economical synthesis have inspired us all and moved the field closer to the ideal.‡Electronic supplementary information (ESI) available: Synthetic procedures and spectral data. See DOI: 10.1039/c4qo00228hClick here for additional data file.Org Chem Front. 2014 Dec 29;1(10):1166-1171. doi: 10.1039/c4qo00228h. Epub 2014 Oct 6. Org Chem Front. 2014. PMID: 25632347 Free PMC article.
-
Toward a biorelevant structure of protein kinase C bound modulators: design, synthesis, and evaluation of labeled bryostatin analogues for analysis with rotational echo double resonance NMR spectroscopy.J Am Chem Soc. 2015 Mar 18;137(10):3678-85. doi: 10.1021/jacs.5b00886. Epub 2015 Mar 4. J Am Chem Soc. 2015. PMID: 25710634 Free PMC article.
-
Reactivity of Thiol-Rich Zn Sites in Diacylglycerol-Sensing PKC C1 Domain Probed by NMR Spectroscopy.Front Mol Biosci. 2021 Aug 10;8:728711. doi: 10.3389/fmolb.2021.728711. eCollection 2021. Front Mol Biosci. 2021. PMID: 34447788 Free PMC article.
References
-
- Alkon DL, Sun MK, Nelson TJ. PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol Sci. 2007;28:51–60. - PubMed
-
- Citron M, Vigo-Pelfrey C, Teplow DB, Miller C, Schenk D, Johnston J, Winblad B, Venizelos N, Lannfelt L, Selkoe DJ. Excessive production of amyloid beta-protein by peripheral cells of symptomatic and presymptomatic patients carrying the Swedish familial Alzheimer disease mutation. Proc Natl Acad Sci USA. 1994;91:11993–11997. - PMC - PubMed
-
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

