T1 weighted brain images at 7 Tesla unbiased for Proton Density, T2* contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization
- PMID: 19233292
- PMCID: PMC2700263
- DOI: 10.1016/j.neuroimage.2009.02.009
T1 weighted brain images at 7 Tesla unbiased for Proton Density, T2* contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization
Abstract
At high magnetic field, MR images exhibit large, undesirable signal intensity variations commonly referred to as "intensity field bias". Such inhomogeneities mostly originate from heterogeneous RF coil B(1) profiles and, with no appropriate correction, are further pronounced when utilizing rooted sum of square reconstruction with receive coil arrays. These artifacts can significantly alter whole brain high resolution T(1)-weighted (T(1)w) images that are extensively utilized for clinical diagnosis, for gray/white matter segmentation as well as for coregistration with functional time series. In T(1) weighted 3D-MPRAGE sequences, it is possible to preserve a bulk amount of T(1) contrast through space by using adiabatic inversion RF pulses that are insensitive to transmit B(1) variations above a minimum threshold. However, large intensity variations persist in the images, which are significantly more difficult to address at very high field where RF coil B(1) profiles become more heterogeneous. Another characteristic of T(1)w MPRAGE sequences is their intrinsic sensitivity to Proton Density and T(2)(*) contrast, which cannot be removed with post-processing algorithms utilized to correct for receive coil sensitivity. In this paper, we demonstrate a simple technique capable of producing normalized, high resolution T(1)w 3D-MPRAGE images that are devoid of receive coil sensitivity, Proton Density and T(2)(*) contrast. These images, which are suitable for routinely obtaining whole brain tissue segmentation at 7 T, provide higher T(1) contrast specificity than standard MPRAGE acquisitions. Our results show that removing the Proton Density component can help in identifying small brain structures and that T(2)(*) induced artifacts can be removed from the images. The resulting unbiased T(1)w images can also be used to generate Maximum Intensity Projection angiograms, without additional data acquisition, that are inherently registered with T(1)w structural images. In addition, we introduce a simple technique to reduce residual signal intensity variations induced by transmit B(1) heterogeneity. Because this approach requires two 3D images, one divided with the other, head motion could create serious problems, especially at high spatial resolution. To alleviate such inter-scan motion problems, we developed a new sequence where the two contrast acquisitions are interleaved within a single scan. This interleaved approach however comes with greater risk of intra-scan motion issues because of a longer single scan time. Users can choose between these two trade offs depending on specific protocols and patient populations. We believe that the simplicity and the robustness of this double contrast based approach to address intensity field bias at high field and improve T(1) contrast specificity, together with the capability of simultaneously obtaining angiography maps, advantageously counter balance the potential drawbacks of the technique, mainly a longer acquisition time and a moderate reduction in signal to noise ratio.
Figures




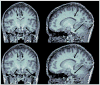
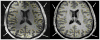




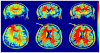

References
-
- Adriany G, Ritter J, Van de Moortele PF, Moeller S, Snyder C, Voje B, Vaughan JT, Ugurbil K. A geometrically adjustable 16 Channel Transceive Transmission Line Array for 7 Tesla. ISMRM 13th Scientific Meeting; Miami. 2005a. p. 673.
-
- Adriany G, Van de Moortele PF, Wiesinger F, Moeller S, Strupp JP, Andersen P, Snyder C, Zhang X, Chen W, Pruessmann KP, Boesiger P, Vaughan T, Ugurbil K. Transmit and receive transmission line arrays for 7 Tesla parallel imaging. Magnetic Resonance in Medicine. 2005b;53:434–445. - PubMed
-
- Belaroussi B, Milles J, Carme S, Zhu YM, Benoit-Cattin H. Intensity non-uniformity correction in MRI: existing methods and their validation. Med Image Anal. 2006;10:234–246. - PubMed
-
- Bernstein MA, Huston J, 3rd, Ward HA. Imaging artifacts at 3.0T. J Magn Reson Imaging. 2006;24:735–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

