Physiological oxidative stress after arousal from hibernation in Arctic ground squirrel
- PMID: 19233307
- PMCID: PMC2784939
- DOI: 10.1016/j.cbpa.2009.02.016
Physiological oxidative stress after arousal from hibernation in Arctic ground squirrel
Abstract
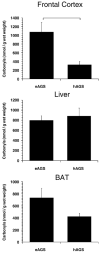
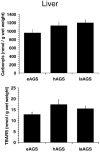
Hibernation in Arctic ground squirrels (AGS), Spermophilus parryii, is characterized by a profound decrease in oxygen consumption and metabolic demand during torpor that is punctuated by periodic rewarming episodes, during which oxygen consumption increases dramatically. The extreme physiology of torpor or the surge in oxygen consumption during arousal may increase production of reactive oxygen species, making hibernation an injurious process for AGS. To determine if AGS tissues experience cellular stress during rewarming, we measured carbonyl proteins, lipid peroxide end products and percent oxidized glutathione in brown adipose tissue (BAT) and liver of torpid, hibernating (hAGS), late arousal (laAGS), and cold-adapted, euthermic AGS (eAGS). In BAT carbonyl proteins and lipid peroxide end products were higher in eAGS and laAGS than in hAGS. By contrast, in liver, no significant difference in carbonyl proteins was observed. In another group of animals, comparison of carbonyl proteins and percent oxidized glutathione in frontal cortex, liver, and BAT of eAGS and hAGS showed no evidence of oxidative stress associated with torpor. These results indicate that increased thermogenesis associated with arousal AGS results in tissue specific oxidative stress in BAT but not in liver. Moreover, torpor per se is largely devoid of oxidative stress, likely due to suppression of oxidative metabolism.
Figures





References
-
- Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu Rev Physiol. 2007;69:145–170. - PubMed
-
- Blagojevic D, Buzadzic B, Korac B, Saicic ZS, Radojicic R, Spasic MB, Petrovic VM. Seasonal changes in the antioxidative defense in ground squirrels (Citellus citellus): possible role of GSH-Px. J Environ Pathol Toxicol Oncol. 1998;17:241–250. - PubMed
-
- Boyer BB, Barnes BM, Lowell BB, Grujic D. Differential regulation of uncoupling protein gene homologues in multiple tissues of hibernating ground squirrels. Am J Physiol. 1998;275:R1232–1238. - PubMed
-
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. - PubMed
-
- Buzadzic B, Spasic M, Saicic ZS, Radojicic R, Petrovic VM, Halliwell B. Antioxidant defenses in the ground squirrel Citellus citellus. 2. The effect of hibernation. Free Radic Biol Med. 1990;9:407–413. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

