Methylation-state-specific recognition of histones by the MBT repeat protein L3MBTL2
- PMID: 19233876
- PMCID: PMC2673432
- DOI: 10.1093/nar/gkp086
Methylation-state-specific recognition of histones by the MBT repeat protein L3MBTL2
Abstract
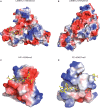
The MBT repeat has been recently identified as a key domain capable of methyl-lysine histone recognition. Functional work has pointed to a role for MBT domain-containing proteins in transcriptional repression of developmental control genes such as Hox genes. In this study, L3MBTL2, a human homolog of Drosophila Sfmbt critical for Hox gene silencing, is demonstrated to preferentially recognize lower methylation states of several histone-derived peptides through its fourth MBT repeat. High-resolution crystallographic analysis of the four MBT repeats of this protein reveals its unique asymmetric rhomboid architecture, as well as binding mechanism, which preclude the interaction of the first three MBT repeats with methylated peptides. Structural elucidation of an L3MBTL2-H4K20me1 complex and comparison with other MBT-histone peptide complexes also suggests that an absence of distinct surface contours surrounding the methyl-lysine-binding pocket may underlie the lack of sequence specificity observed for members of this protein family.
Figures

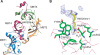

References
-
- Grewal SI, Jia S. Heterochromatin revisited. Nat. Rev. 2007;8:35–46. - PubMed
-
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Gen. Dev. 2004;14:155–164. - PubMed
-
- Jacobs SA, Khorasanizadeh S. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science. 2002;295:2080–2083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

