The tubulin-like RepX protein encoded by the pXO1 plasmid forms polymers in vivo in Bacillus anthracis
- PMID: 19233922
- PMCID: PMC2668384
- DOI: 10.1128/JB.00027-09
The tubulin-like RepX protein encoded by the pXO1 plasmid forms polymers in vivo in Bacillus anthracis
Abstract
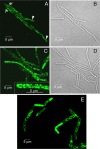
Bacillus anthracis contains two megaplasmids, pXO1 and pXO2, that are critical for its pathogenesis. Stable inheritance of pXO1 in B. anthracis is dependent upon the tubulin/FtsZ-like RepX protein encoded by this plasmid. Previously, we have shown that RepX undergoes GTP-dependent polymerization in vitro. However, the polymerization properties and localization pattern of RepX in vivo are not known. Here, we utilize a RepX-green fluorescent protein (GFP) fusion to show that RepX forms foci and three distinct forms of polymeric structures in B. anthracis in vivo, namely straight, curved, and helical filaments. Polymerization of RepX-GFP as well as the nature of polymers formed were dependent upon concentration of the protein inside the B. anthracis cells. RepX predominantly localized as polymers that were parallel to the length of the cell. RepX also formed polymers in Escherichia coli in the absence of other pXO1-encoded products, showing that in vivo polymerization is an inherent property of the protein and does not require either the pXO1 plasmid or proteins unique to B. anthracis. Overexpression of RepX did not affect the cell morphology of B. anthracis cells, whereas it drastically distorted the cell morphology of E. coli host cells. We discuss the significance of our observations in view of the plasmid-specific functions that have been proposed for RepX and related proteins encoded by several megaplasmids found in members of the Bacillus cereus group of bacteria.
Figures
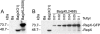


References
-
- Anand, S. P., P. Akhtar, E. Tinsley, S. C. Watkins, and S. A. Khan. 2008. GTP-dependent polymerization of the tubulin-like RepX replication protein encoded by the pXO1 plasmid of Bacillus anthracis. Mol. Microbiol. 67881-890. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

