An essential role for the RNA-binding protein Smaug during the Drosophila maternal-to-zygotic transition
- PMID: 19234062
- PMCID: PMC2727558
- DOI: 10.1242/dev.031815
An essential role for the RNA-binding protein Smaug during the Drosophila maternal-to-zygotic transition
Abstract
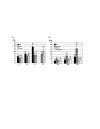
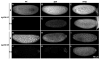
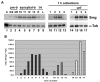
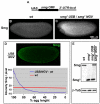
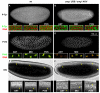
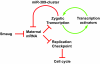
Genetic control of embryogenesis switches from the maternal to the zygotic genome during the maternal-to-zygotic transition (MZT), when maternal mRNAs are destroyed, high-level zygotic transcription is initiated, the replication checkpoint is activated and the cell cycle slows. The midblastula transition (MBT) is the first morphological event that requires zygotic gene expression. The Drosophila MBT is marked by blastoderm cellularization and follows 13 cleavage-stage divisions. The RNA-binding protein Smaug is required for cleavage-independent maternal transcript destruction during the Drosophila MZT. Here, we show that smaug mutants also disrupt syncytial blastoderm stage cell-cycle delays, DNA replication checkpoint activation, cellularization, and high-level zygotic expression of protein coding and micro RNA genes. We also show that Smaug protein levels increase through the cleavage divisions and peak when the checkpoint is activated and zygotic transcription initiates, and that transgenic expression of Smaug in an anterior-to-posterior gradient produces a concomitant gradient in the timing of maternal transcript destruction, cleavage cell cycle delays, zygotic gene transcription, cellularization and gastrulation. Smaug accumulation thus coordinates progression through the MZT.
Figures


References
-
- Abdu, U., Brodsky, M. and Schupbach, T. (2002). Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr. Biol. 12, 1645-1651. - PubMed
-
- Bashirullah, A., Halsell, S. R., Cooperstock, R. L., Kloc, M., Karaiskakis, A., Fisher, W. W., Fu, W., Hamilton, J. K., Etkin, L. D. and Lipshitz, H. D. (1999). Joint action of two RNA degradation pathways controls the timing of maternal transcript elimination at the midblastula transition in Drosophila melanogaster. EMBO J. 18, 2610-2620. - PMC - PubMed
-
- Bushati, N., Stark, A., Brennecke, J. and Cohen, S. M. (2008). Temporal reciprocity of miRNAs and their targets during the maternal-to-zygotic transition in Drosophila. Curr. Biol. 18, 501-506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

