Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis
- PMID: 19234125
- PMCID: PMC2656197
- DOI: 10.1073/pnas.0900196106
Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis
Abstract
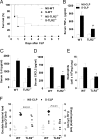
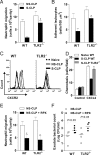
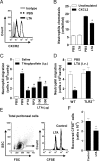
Patients with sepsis have a marked defect in neutrophil migration. Here we identify a key role of Toll-like receptor 2 (TLR2) in the regulation of neutrophil migration and resistance during polymicrobial sepsis. We found that the expression of the chemokine receptor CXCR2 was dramatically down-regulated in circulating neutrophils from WT mice with severe sepsis, which correlates with reduced chemotaxis to CXCL2 in vitro and impaired migration into an infectious focus in vivo. TLR2 deficiency prevented the down-regulation of CXCR2 and failure of neutrophil migration. Moreover, TLR2(-/-) mice exhibited higher bacterial clearance, lower serum inflammatory cytokines, and improved survival rate during severe sepsis compared with WT mice. In vitro, the TLR2 agonist lipoteichoic acid (LTA) down-regulated CXCR2 expression and markedly inhibited the neutrophil chemotaxis and actin polymerization induced by CXCL2. Moreover, neutrophils activated ex vivo by LTA and adoptively transferred into naïve WT recipient mice displayed a significantly reduced competence to migrate toward thioglycolate-induced peritonitis. Finally, LTA enhanced the expression of G protein-coupled receptor kinases 2 (GRK2) in neutrophils; increased expression of GRK2 was seen in blood neutrophils from WT mice, but not TLR2(-/-) mice, with severe sepsis. Our findings identify an unexpected detrimental role of TLR2 in polymicrobial sepsis and suggest that inhibition of TLR2 signaling may improve survival from sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
-
- Benjamim CF, Ferreira SH, Cunha FdQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis. 2000;182:214–223. - PubMed
-
- Orinska Z, et al. IL-15 constrains mast cell–dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13:927–934. - PubMed
-
- Alves-Filho JC, et al. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl 1):3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

