Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR
- PMID: 19234131
- PMCID: PMC2656159
- DOI: 10.1073/pnas.0804543106
Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR
Abstract
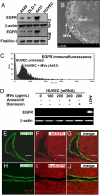
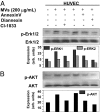
Activated EGF receptor (EGFR) plays an oncogenic role in several human malignancies. Although the intracellular effects of EGFR are well studied, its ability to induce and modulate tumor angiogenesis is less understood. We found previously that oncogenic EGFR can be shed from cancer cells as cargo of membrane microvesicles (MVs), which can interact with surfaces of other cells. Here we report that MVs produced by human cancer cells harboring activated EGFR (A431, A549, DLD-1) can be taken up by cultured endothelial cells, in which they elicit EGFR-dependent responses, including activation of MAPK and Akt pathways. These responses can be blocked by annexin V and its homodimer, Diannexin, both of which cloak phosphatidylserine residues on the surfaces of MVs. Interestingly, the intercellular EGFR transfer is also accompanied by the onset of VEGF expression in endothelial cells and by autocrine activation of its key signaling receptor (VEGF receptor-2). In A431 human tumor xenografts in mice, angiogenic endothelial cells stain positively for human EGFR and phospho-EGFR, while treatment with Diannexin leads to a reduction of tumor growth rate and microvascular density. Thus, we propose that oncogene-containing tumor cell-derived MVs could act as a unique form of angiogenesis-modulating stimuli and are capable of switching endothelial cells to act in an autocrine mode.
Conflict of interest statement
Conflict of interest statement: A.C.A. is employed by Alavita, which develops therapeutic applications of Diannexin, mainly in cardiovascular medicine.
Figures


References
-
- Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. - PubMed
-
- Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005;27:375–387. - PubMed
-
- Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol Dis. 2006;36:315–321. - PubMed
-
- Janowska-Wieczorek A, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. %20; - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

