Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype
- PMID: 19234137
- PMCID: PMC2665768
- DOI: 10.2353/ajpath.2009.080431
Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype
Abstract
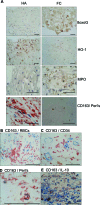
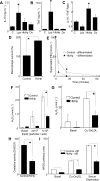
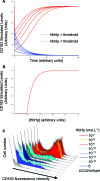
Intraplaque hemorrhage accelerates atherosclerosis via oxidant stress and contributes to lesion development and destabilization. Normally, macrophages scavenge hemoglobin-haptoglobin (HbHp) complexes via CD163, and this process provokes the secretion of the anti-inflammatory atheroprotective cytokine interleukin (IL)-10. We therefore tested the hypothesis that HbHp complexes may drive monocyte differentiation to an atheroprotective phenotype. Examination of the macrophage phenotype in hemorrhaged atherosclerotic plaques revealed a novel hemorrhage-associated macrophage population (HA-mac), defined by high levels of CD163, but low levels of human leukocyte antigen-DR. HA-mac contained more iron, a pro-oxidant catalyst, but paradoxically had less oxidative injury, measured by 8-oxo-guanosine content. Differentiating monocytes with HbHp complexes reproduced the CD163(high) human leukocyte antigen-DR(low) HA-mac phenotype in vitro. These in vitro HA-mac cells cleared Hb more quickly, and consistently showed less hydrogen peroxide release, highly reactive oxygen species and oxidant stress, and increased survival. Differentiation to HA-mac was prevented by neutralizing IL-10 antibodies, indicating that IL-10 mediates an autocrine feedback mechanism in this system. Nonlinear dynamic modeling showed that an IL-10/CD163-positive feedback loop drove a discrete HA-mac lineage. Simulations further indicated an all-or-none switch to HA-mac at threshold levels of HbHp, and this conversion was experimentally verified. These data demonstrate the creation of a novel atheroprotective (HA-mac) macrophage subpopulation in response to intraplaque hemorrhage and raise the possibility that therapeutically reproducing this macrophage phenotype may be cardio-protective in cases of atherosclerosis.
Figures


References
-
- Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. - PubMed
-
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. - PubMed
-
- Levy AP, Moreno PR. Intraplaque hemorrhage. Curr Mol Med. 2006;6:479–488. - PubMed
-
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. - PubMed
-
- Lin HL, Xu XS, Lu HX, Zhang L, Li CJ, Tang MX, Sun HW, Liu Y, Zhang Y. Pathological mechanisms and dose dependency of erythrocyte-induced vulnerability of atherosclerotic plaques. J Mol Cell Cardiol. 2007;43:272–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

