Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation
- PMID: 19234157
- PMCID: PMC4291126
- DOI: 10.4049/jimmunol.0802954
Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation
Abstract
In the CNS, the transcription factor NF-kappaB is a key regulator of inflammation and secondary injury processes. Following trauma or disease, the expression of NF-kappaB-dependent genes is activated, leading to both protective and detrimental effects. In this study, we show that transgenic inactivation of astroglial NF-kappaB (glial fibrillary acidic protein-IkappaB alpha-dominant-negative mice) resulted in reduced disease severity and improved functional recovery following experimental autoimmune encephalomyelitis. At the chronic stage of the disease, transgenic mice exhibited an overall higher presence of leukocytes in spinal cord and brain, and a markedly higher percentage of CD8(+)CD122(+) T regulatory cells compared with wild type, which correlated with the timing of clinical recovery. We also observed that expression of proinflammatory genes in both spinal cord and cerebellum was delayed and reduced, whereas the loss of neuronal-specific molecules essential for synaptic transmission was limited compared with wild-type mice. Furthermore, death of retinal ganglion cells in affected retinas was almost abolished, suggesting the activation of neuroprotective mechanisms. Our data indicate that inhibiting NF-kappaB in astrocytes results in neuroprotective effects following experimental autoimmune encephalomyelitis, directly implicating astrocytes in the pathophysiology of this disease.
Figures







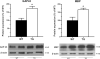
References
-
- Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 2000;157:267–276. - PMC - PubMed
-
- Ayers MM, Hazelwood LJ, Catmull DV, Wang D, McKormack Q, Bernard CC, Orian JM. Early glial responses in murine models of multiple sclerosis. Neurochem. Int. 2004;45:409–419. - PubMed
-
- Bjartmar C, Trapp BD. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr. Opin. Neurol. 2001;14:271–278. - PubMed
-
- Wujek JR, Bjartmar C, Richer E, Ransohoff RM, Yu M, Tuohy VK, Trapp BD. Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J. Neuropathol. Exp. Neurol. 2002;61:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

