Selective priming and expansion of antigen-specific Foxp3- CD4+ T cells during Listeria monocytogenes infection
- PMID: 19234199
- PMCID: PMC2677098
- DOI: 10.4049/jimmunol.0803402
Selective priming and expansion of antigen-specific Foxp3- CD4+ T cells during Listeria monocytogenes infection
Abstract
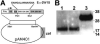
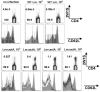
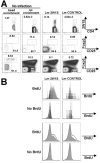
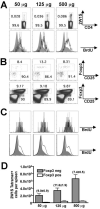
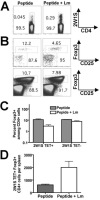
The Foxp3-expressing subset of regulatory CD4(+) T cells have defined Ag specificity and play essential roles in maintaining peripheral tolerance by suppressing the activation of self-reactive T cells. Similarly, during chronic infection, pathogen-specific Foxp3-expressing CD4(+) T cells expand and actively suppress pathogen-specific effector T cells. Herein, we used MHC class II tetramers and Foxp3(gfp) knockin mice to track the kinetics and magnitude whereby pathogen-specific Foxp3(+)CD4(+) and Foxp3(-)CD4(+) cells are primed and expand after acute infection with recombinant Listeria monocytogenes (Lm) expressing the non-"self"-Ag 2W1S(52-68). We demonstrate that Lm infection selectively primes proliferation, expansion, and subsequent contraction of Lm-specific Foxp3(-) effector CD4(+) cells, while the numbers of Lm-specific Foxp3(+)CD4(+) regulatory cells remain essentially unchanged. In sharp contrast, purified 2W1S(52-68) peptide primes coordinated expansion of both Foxp3(+) regulatory and Foxp3(-) effector T cells with the same Ag specificity. Taken together, these results indicate selective priming and expansion of Foxp3(-) CD4 T cells is a distinguishing feature for acute bacterial infection.
Figures

References
-
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. - PubMed
-
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. - PubMed
-
- Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. - PubMed
-
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

