Domain architecture of the stator complex of the A1A0-ATP synthase from Thermoplasma acidophilum
- PMID: 19234304
- PMCID: PMC2673272
- DOI: 10.1074/jbc.M808962200
Domain architecture of the stator complex of the A1A0-ATP synthase from Thermoplasma acidophilum
Abstract
A key structural element in the ion translocating F-, A-, and V-ATPases is the peripheral stalk, an assembly of two polypeptides that provides a structural link between the ATPase and ion channel domains. Previously, we have characterized the peripheral stalk forming subunits E and H of the A-ATPase from Thermoplasma acidophilum and demonstrated that the two polypeptides interact to form a stable heterodimer with 1:1 stoichiometry (Kish-Trier, E., Briere, L. K., Dunn, S. D., and Wilkens, S. (2008) J. Mol. Biol. 375, 673-685). To define the domain architecture of the A-ATPase peripheral stalk, we have now generated truncated versions of the E and H subunits and analyzed their ability to bind each other. The data show that the N termini of the subunits form an alpha-helical coiled-coil, approximately 80 residues in length, whereas the C-terminal residues interact to form a globular domain containingalpha- and beta-structure. We find that the isolated C-terminal domain of the E subunit exists as a dimer in solution, consistent with a recent crystal structure of the related Pyrococcus horikoshii A-ATPase E subunit (Lokanath, N. K., Matsuura, Y., Kuroishi, C., Takahashi, N., and Kunishima, N. (2007) J. Mol. Biol. 366, 933-944). However, upon the addition of a peptide comprising the C-terminal 21 residues of the H subunit (or full-length H subunit), dimeric E subunit C-terminal domain dissociates to form a 1:1 heterodimer. NMR spectroscopy was used to show that H subunit C-terminal peptide binds to E subunit C-terminal domain via the terminal alpha-helices, with little involvement of the beta-sheet region. Based on these data, we propose a structural model of the A-ATPase peripheral stalk.
Figures

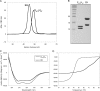
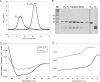
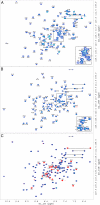

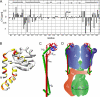
References
-
- Forgac, M. (2007) Nat. Rev. Mol. Cell Biol. 8 917-929 - PubMed
-
- Boyer, P. D. (1997) Annu. Rev. Biochem. 66 717-749 - PubMed
-
- Wilkens, S. (2005) Adv. Prot. Chem. 71 345-382 - PubMed
-
- Coskun, U., Chaban, Y. L., Lingl, A., Müller, V., Keegstra, W., Boekema, E. J., and Grüber, G. (2004) J. Biol. Chem. 279 38644-38648 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

