Membrane scission by the ESCRT-III complex
- PMID: 19234443
- PMCID: PMC2743992
- DOI: 10.1038/nature07836
Membrane scission by the ESCRT-III complex
Abstract
The endosomal sorting complex required for transport (ESCRT) system is essential for multivesicular body biogenesis, in which cargo sorting is coupled to the invagination and scission of intralumenal vesicles. The ESCRTs are also needed for budding of enveloped viruses including human immunodeficiency virus 1, and for membrane abscission in cytokinesis. In Saccharomyces cerevisiae, ESCRT-III consists of Vps20, Snf7, Vps24 and Vps2 (also known as Did4), which assemble in that order and require the ATPase Vps4 for their disassembly. In this study, the ESCRT-III-dependent budding and scission of intralumenal vesicles into giant unilamellar vesicles was reconstituted and visualized by fluorescence microscopy. Here we show that three subunits of ESCRT-III, Vps20, Snf7 and Vps24, are sufficient to detach intralumenal vesicles. Vps2, the ESCRT-III subunit responsible for recruiting Vps4, and the ATPase activity of Vps4 were required for ESCRT-III recycling and supported additional rounds of budding. The minimum set of ESCRT-III and Vps4 proteins capable of multiple cycles of vesicle detachment corresponds to the ancient set of ESCRT proteins conserved from archaea to animals.
Figures
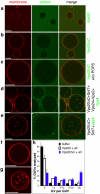
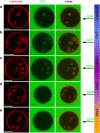

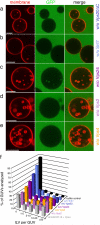
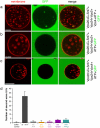
Comment in
-
Cell biology: Detached membrane bending.Nature. 2009 Mar 12;458(7235):159-60. doi: 10.1038/458159a. Nature. 2009. PMID: 19279626 No abstract available.
References
-
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat. Rev. Mol. Cell Biol. 2004;5:317–323. - PubMed
-
- Russell MRG, Nickerson DP, Odorizzi G. Molecular mechanisms of late endosome morphology, identity and sorting. Curr. Opin. Cell Biol. 2006;18:422–428. - PubMed
-
- Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and nonendosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. - PubMed
-
- Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem. Sci. 2007;32:561–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

