Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10
- PMID: 19234462
- PMCID: PMC2693210
- DOI: 10.1038/nm.1929
Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10
Abstract
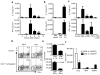
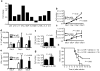
Activated antigen-specific T cells produce a variety of effector molecules for clearing infection but also contribute to inflammation and tissue injury. Here we report an anti-inflammatory property of antiviral CD8+ and CD4+ effector T cells (T(eff) cells) in the infected periphery during acute virus infection. We find that, during acute influenza infection, interleukin-10 (IL-10) is produced in the infected lungs in large amounts--exclusively by infiltrating virus-specific T(eff) cells, with CD8+ T(eff) cells contributing a larger fraction of the IL-10 produced. These T(eff) cells in the periphery simultaneously produce IL-10 and proinflammatory cytokines and express lineage markers characteristic of conventional T helper type 1 or T cytotoxic type 1 cells. Notably, blocking the action of the T(eff) cell-derived IL-10 results in enhanced pulmonary inflammation and lethal injury. Our results show that antiviral T(eff) cells exert regulatory functions--that is, they fine-tune the extent of lung inflammation and injury associated with influenza infection by producing an anti-inflammatory cytokine. We discuss the potential implications of these findings for infection with highly pathogenic influenza viruses.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures




References
-
- Cheung CY, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–7. - PubMed
-
- Kobasa D, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–23. - PubMed
-
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. - PubMed
-
- Couper KN, Blount DG, Riley EM. IL-10: The Master Regulator of Immunity to Infection. J Immunol. 2008;180:5771–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

