ABC transporters: the power to change
- PMID: 19234479
- PMCID: PMC2830722
- DOI: 10.1038/nrm2646
ABC transporters: the power to change
Abstract
ATP-binding cassette (ABC) transporters constitute a ubiquitous superfamily of integral membrane proteins that are responsible for the ATP-powered translocation of many substrates across membranes. The highly conserved ABC domains of ABC transporters provide the nucleotide-dependent engine that drives transport. By contrast, the transmembrane domains that create the translocation pathway are more variable. Recent structural advances with prokaryotic ABC transporters have provided a qualitative molecular framework for deciphering the transport cycle. An important goal is to develop quantitative models that detail the kinetic and molecular mechanisms by which ABC transporters couple the binding and hydrolysis of ATP to substrate translocation.
Conflict of interest statement
The authors declare no competing financial interests
Figures


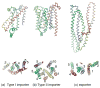

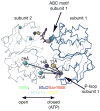
References
-
- Monod J. Recherches sur la croissance des culture bactériennes. Hermann, Editeurs des Sciences et des Arts; Paris: 1942.
-
- Phillips R, Quake SR. The biological frontier of physics. Physics Today. 2006;59:38–43.
-
- Blattner FR, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. - PubMed
-
- Busch W, Saier MHJ. The transporter classification (TC) system. Crit Rev Biochem Mol Biol. 2002;27:287–337. - PubMed
-
- Stouthamer AH. The search for correlation between theoretical and experimental growth yields. Int Rev Biochem. 1979;21:1–47.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

