Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes
- PMID: 19235890
- PMCID: PMC2911960
- DOI: 10.1002/jnr.22029
Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes
Abstract
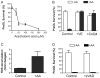
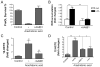
Oxidative mechanisms of injury are important in many neurological disorders. Developing oligodendrocytes (pre-OLs) are particularly sensitive to oxidative stress-mediated injury. We previously demonstrated a novel function of phylloquinone (vitamin K(1)) and menaquinone 4 (MK-4; a major form of vitamin K2) in protecting pre-OLs and immature neurons against glutathione depletion-induced oxidative damage (Li et al. [ 2003] J. Neurosci. 23:5816-5826). Here we report that vitamin K at nanomolar concentrations prevents arachidonic acid-induced oxidative injury to pre-OLs through blocking the activation of 12-lipoxygenase (12-LOX). Arachidonic acid metabolism is a potential source for reactive oxygen species (ROS) generation during ischemia and reperfusion. Exposure of pre-OLs to arachidonic acid resulted in oxidative cell death in a concentration-dependent manner. Administration of vitamin K (K(1) and MK-4) completely prevented the toxicity. Consistent with our previous findings, inhibitors of 12-LOX abolished ROS production and cell death, indicating that activation of 12-LOX is a key event in arachidonic acid-induced pre-OL death. Vitamin K(1) and MK-4 significantly blocked 12-LOX activation and prevented ROS accumulation in pre-OLs challenged with arachidonic acid. However, vitamin K itself did not directly inhibit 12-LOX enzymatic activity when assayed with purified 12-LOX in vitro. These results suggest that vitamin K, or likely its metabolites, acts upstream of activation of 12-LOX in pre-OLs. In summary, our data indicate that vitamin K prevents oxidative cell death by blocking activation of 12-LOX and ROS generation.
(c) 2009 Wiley-Liss, Inc.
Figures



Similar articles
-
12/15-lipoxygenase expression is increased in oligodendrocytes and microglia of periventricular leukomalacia.Dev Neurosci. 2013;35(2-3):140-54. doi: 10.1159/000350230. Epub 2013 Apr 20. Dev Neurosci. 2013. PMID: 23838566 Free PMC article.
-
12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes.Eur J Neurosci. 2004 Oct;20(8):2049-58. doi: 10.1111/j.1460-9568.2004.03650.x. Eur J Neurosci. 2004. PMID: 15450084
-
Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons.J Neurosci. 2003 Jul 2;23(13):5816-26. doi: 10.1523/JNEUROSCI.23-13-05816.2003. J Neurosci. 2003. PMID: 12843286 Free PMC article.
-
Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species.BMB Rep. 2008 Aug 31;41(8):555-9. doi: 10.5483/bmbrep.2008.41.8.555. BMB Rep. 2008. PMID: 18755069 Review.
-
Intracellular zinc release, 12-lipoxygenase activation and MAPK dependent neuronal and oligodendroglial death.Mol Med. 2007 Jul-Aug;13(7-8):350-5. doi: 10.2119/2007–00042.Zhang. Mol Med. 2007. PMID: 17622306 Free PMC article. Review.
Cited by
-
The biological responses of vitamin K2: A comprehensive review.Food Sci Nutr. 2023 Jan 6;11(4):1634-1656. doi: 10.1002/fsn3.3213. eCollection 2023 Apr. Food Sci Nutr. 2023. PMID: 37051359 Free PMC article. Review.
-
12/15-lipoxygenase expression is increased in oligodendrocytes and microglia of periventricular leukomalacia.Dev Neurosci. 2013;35(2-3):140-54. doi: 10.1159/000350230. Epub 2013 Apr 20. Dev Neurosci. 2013. PMID: 23838566 Free PMC article.
-
Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases.Nutrients. 2020 Jan 3;12(1):138. doi: 10.3390/nu12010138. Nutrients. 2020. PMID: 31947821 Free PMC article. Review.
-
Diabetes mellitus: channeling care through cellular discovery.Curr Neurovasc Res. 2010 Feb;7(1):59-64. doi: 10.2174/156720210790820217. Curr Neurovasc Res. 2010. PMID: 20158461 Free PMC article.
-
A novel locus in the oxidative stress-related gene ALOX12 moderates the association between PTSD and thickness of the prefrontal cortex.Psychoneuroendocrinology. 2015 Dec;62:359-65. doi: 10.1016/j.psyneuen.2015.09.003. Epub 2015 Sep 5. Psychoneuroendocrinology. 2015. PMID: 26372769 Free PMC article.
References
-
- Back SA, Khan R, Gan X, Rosenberg PA, Volpe JJ. A new alamar blue viability assay to rapidly quantify oligodendrocyte death. J Neurosci Methods. 1999;91:47–54. - PubMed
-
- Back SA, Luo NL, Mallinson RA, O’Malley JP, Wallen LD, Frei B, Morrow JD, Petito CK, Roberts CT, Jr, Murdoch GH, Montine TJ. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58:108–120. - PubMed
-
- Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. - PubMed
-
- Bendani MK, Palluy O, Cook-Moreau J, Beneytout JL, Rigaud M, Vallat JM. Localization of 12-lipoxygenase mRNA in cultured oligodendrocytes and astrocytes by in situ reverse transcriptase and polymerase chain reaction. Neurosci Lett. 1995;189:159–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

