Human immature dental pulp stem cells' contribution to developing mouse embryos: production of human/mouse preterm chimaeras
- PMID: 19236382
- PMCID: PMC6496747
- DOI: 10.1111/j.1365-2184.2008.00578.x
Human immature dental pulp stem cells' contribution to developing mouse embryos: production of human/mouse preterm chimaeras
Abstract
Objectives: In this study, we aimed at determining whether human immature dental pulp stem cells (hIDPSC) would be able to contribute to different cell types in mouse blastocysts without damaging them. Also, we analysed whether these blastocysts would progress further into embryogenesis when implanted to the uterus of foster mice, and develop human/mouse chimaera with retention of hIDPSC derivates and their differentiation.
Materials and methods: hIDPSC and mouse blastocysts were used in this study. Fluorescence staining of hIDPSC and injection into mouse blastocysts, was performed. Histology, immunohistochemistry, fluorescence in situ hybridization and confocal microscopy were carried out.
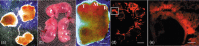
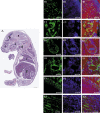
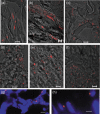
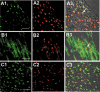
Results and conclusion: hIDPSC showed biological compatibility with the mouse host environment and could survive, proliferate and contribute to the inner cell mass as well as to the trophoblast cell layer after introduction into early mouse embryos (n = 28), which achieved the hatching stage following 24 and 48 h in culture. When transferred to foster mice (n = 5), these blastocysts with hIDPSC (n = 57) yielded embryos (n = 3) and foetuses (n = 6); demonstrating presence of human cells in various organs, such as brain, liver, intestine and hearts, of the human/mouse chimaeras. We verified whether hIDPSC would also be able to differentiate into specific cell types in the mouse environment. Contribution of hIDPSC in at least two types of tissues (muscles and epithelial), was confirmed. We showed that hIDPSC survived, proliferated and differentiated in mouse developing blastocysts and were capable of producing human/mouse chimaeras.
Figures

References
-
- Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156. - PubMed
-
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jeffrey M (1998) Jones Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. - PubMed
-
- Smith A (2001) Embryonic stem cells In: Marshak DR, Gardner RL, Gottlieb D, eds. Stem Cell Biology, pp. 205–230, Cold Spring Harbor, NY: Cold Spring; Harbor Laboratory Press.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

