Alpha-fluoro-alpha-nitro(phenylsulfonyl)methane as a fluoromethyl pronucleophile: efficient stereoselective Michael addition to chalcones
- PMID: 19237559
- PMCID: PMC2657402
- DOI: 10.1073/pnas.0900179106
Alpha-fluoro-alpha-nitro(phenylsulfonyl)methane as a fluoromethyl pronucleophile: efficient stereoselective Michael addition to chalcones
Abstract
Highly efficient stereoselective 1,4-addition of racemic alpha-fluoro-alpha-nitro(phenylsulfonyl)methane (FNSM) as a fluoromethyl pronucleophile to alpha,beta-unsaturated ketones using a wide range of chiral organobifunctional catalysts under moderate conditions in the absence of an additional base has been achieved. A series of catalysts was screened for the enantioselective addition of FNSM to chalcones and the catalysts CN I, CD I, QN I-IV, and QD I were found to enable this reaction, successfully providing exclusive 1,4-addition products stereoselectively in high yields (conversion, diastereomeric ratio, and enantiomeric excess). Studies involving a model reaction and systematic analysis of the absolute configuration support the suggested mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

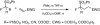



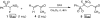

References
-
- Kirsh P. Modern Fluoroorganic Chemistry. Weinheim, Germany: Wiley-VCH; 2004.
-
- Smart BE. Fluorine substituent effects (on bioactivity) J Fluorine Chem. 2001;109:3–11.
-
- Soloshonok VA. Enantiocontrolled Synthesis of Fluoroorganic Compounds: Stereochemical Challenges and Biomedical Targets. Chichester, UK: Wiley-VCH; 1999.
-
- Banks RE, Smart BE, Tatlow JC. Organofluorine Chemistry: Principles and Commercial Applications. New York: Plenum; 1994.
-
- Filler R, Kobayashim Y, Yagupolski LM. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications. Amsterdam: Elsevier; 1993.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

