Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration
- PMID: 19237597
- PMCID: PMC2654119
- DOI: 10.1083/jcb.200808042
Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration
Abstract
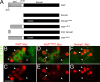
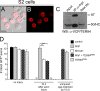
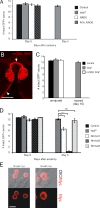
Slow Wallerian degeneration (Wld(S)) encodes a chimeric Ube4b/nicotinamide mononucleotide adenylyl transferase 1 (Nmnat1) fusion protein that potently suppresses Wallerian degeneration, but the mechanistic action of Wld(S) remains controversial. In this study, we characterize Wld(S)-mediated axon protection in vivo using Drosophila melanogaster. We show that Nmnat1 can protect severed axons from autodestruction but at levels significantly lower than Wld(S), and enzyme-dead versions of Nmnat1 and Wld(S) exhibit severely reduced axon-protective function. Interestingly, a 16-amino acid N-terminal domain of Wld(S) (termed N16) accounts for the differences in axon-sparing activity between Wld(S) and Nmnat1, and N16-dependent enhancement of Nmnat1-protective activity in Wld(S) requires the N16-binding protein valosin-containing protein (VCP)/TER94. Thus, Wld(S)-mediated suppression of Wallerian degeneration results from VCP-N16 interactions and Nmnat1 activity converging in vivo. Surprisingly, mouse Nmnat3, a mitochondrial Nmnat enzyme that localizes to the cytoplasm in Drosophila cells, protects severed axons at levels indistinguishable from Wld(S). Thus, nuclear Nmnat activity does not appear to be essential for Wld(S)-like axon protection.
Figures


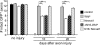

References
-
- Araki T., Sasaki Y., Milbrandt J. 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration.Science. 305:1010–1013 - PubMed
-
- Bittner G.D. 1991. Long-term survival of anucleate axons and its implications for nerve regeneration.Trends Neurosci. 14:188–193 - PubMed
-
- Coleman M. 2005. Axon degeneration mechanisms: commonality amid diversity.Nat. Rev. Neurosci. 6:889–898 - PubMed
-
- Coleman M.P., Perry V.H. 2002. Axon pathology in neurological disease: a neglected therapeutic target.Trends Neurosci. 25:532–537 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

