Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity
- PMID: 19237601
- PMCID: PMC2699126
- DOI: 10.1084/jem.20082394
Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity
Abstract
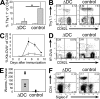
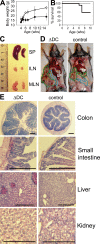
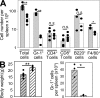
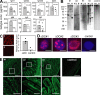
Lack of immunological tolerance against self-antigens results in autoimmune disorders. During onset of autoimmunity, dendritic cells (DCs) are thought to be critical for priming of self-reactive T cells that have escaped tolerance induction. However, because DCs can also induce T cell tolerance, it remains unclear whether DCs are required under steady-state conditions to prevent autoimmunity. To address this question, we crossed CD11c-Cre mice with mice that express diphtheria toxin A (DTA) under the control of a loxP-flanked neomycin resistance (neo(R)) cassette from the ROSA26 locus. Cre-mediated removal of the neo(R) cassette leads to DTA expression and constitutive loss of conventional DCs, plasmacytoid DCs, and Langerhans cells. These DC-depleted (DeltaDC) mice showed increased frequencies of CD4 single-positive thymocytes and infiltration of CD4 T cells into peripheral tissues. They developed spontaneous autoimmunity characterized by reduced body weight, splenomegaly, autoantibody formation, neutrophilia, high numbers of Th1 and Th17 cells, and inflammatory bowel disease. Pathology could be induced by reconstitution of wild-type (WT) mice with bone marrow (BM) from DeltaDC mice, whereas mixed BM chimeras that received BM from DeltaDC and WT mice remained healthy. This demonstrates that DCs play an essential role to protect against fatal autoimmunity under steady-state conditions.
Figures




References
-
- Anderson G., Moore N.C., Owen J.J., Jenkinson E.J. 1996. Cellular interactions in thymocyte development.Annu. Rev. Immunol. 14:73–99 - PubMed
-
- Laufer T.M., DeKoning J., Markowitz J.S., Lo D., Glimcher L.H. 1996. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex.Nature. 383:81–85 - PubMed
-
- Volkmann A., Zal T., Stockinger B. 1997. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen.J. Immunol. 158:693–706 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

