Early postpartum pharmacokinetics of lopinavir initiated intrapartum in Thai women
- PMID: 19237646
- PMCID: PMC2681511
- DOI: 10.1128/AAC.01091-08
Early postpartum pharmacokinetics of lopinavir initiated intrapartum in Thai women
Abstract
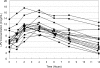
Lopinavir (LPV) exposure is reduced during the third trimester of pregnancy. We report the pharmacokinetics of standard LPV-ritonavir dosing (400/100 mg twice daily) in the immediate and early postpartum period when initiated during labor. In 16 human immunodeficiency virus-infected Thai women, the median (range) LPV area under the concentration-time curve and maximum and minimum concentrations in plasma were 99.7 (66.1 to 180.5) microg x h/ml, 11.2 (8.0 to 17.5) microg/ml, and 4.6 (1.7 to 12.5) microg/ml, respectively, at 41 (12 to 74) h after delivery. All of the women attained adequate LPV levels through 30 days postpartum. No serious adverse events were reported.
Figures
References
-
- Chi, B. H., M. Sinkala, F. Mbewe, R. A. Cantrell, G. Kruse, N. Chintu, G. M. Aldrovandi, E. M. Stringer, C. Kankasa, J. T. Safrit, and J. S. Stringer. 2007. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet 370:1698-1705. - PubMed
-
- Droste, J. A., C. P. Verweij-Van Wissen, and D. M. Burger. 2003. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther. Drug Monit. 25:393-399. - PubMed
-
- Gibbons, S., D. Back, and S. Khoo. 2007. Variability in lopinavir concentrations in the clinical setting and factors affecting concentrations, abstr. 37. Eighth International Workshop on Clinical Pharmacology of HIV Infection, Budapest, Hungary, April 2007.
-
- McIntyre, J., N. Martinson, Investigators for the Trial 1413, V. Boltz, S. Palmer, J. Coffin, J. Mellors, M. Hopley, T. Kimura, P. Robinson, and D. Mayers. 2004. Addition of short course combivir (CBV) to single dose viramune (sdNVP) for prevention of mother-to-child transmission (MTCT) of HIV-1 can significantly decrease the subsequent development of maternal NNRTI-resistant virus, abstr. LbOrB09. XV International AIDS Conference, Bangkok, Thailand, 11 to 16 July 2004.
-
- Murphy, R. L., S. Brun, C. Hicks, J. J. Eron, R. Gulick, M. King, A. C. White, Jr., C. Benson, M. Thompson, H. A. Kessler, S. Hammer, R. Bertz, A. Hsu, A. Japour, and E. Sun. 2001. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS 15:F1-F9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical