Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy
- PMID: 19237662
- PMCID: PMC2703676
- DOI: 10.1161/CIRCULATIONAHA.108.794834
Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy
Abstract
Background: Cardiac resynchronization therapy (CRT) is widely applied in patients with heart failure and dyssynchronous contraction (DHF), but the electrophysiological consequences of CRT in heart failure remain largely unexplored.
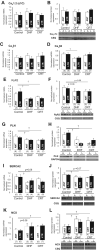
Methods and results: Adult dogs underwent left bundle-branch ablation and either right atrial pacing (190 to 200 bpm) for 6 weeks (DHF) or 3 weeks of right atrial pacing followed by 3 weeks of resynchronization by biventricular pacing at the same pacing rate (CRT). Isolated left ventricular anterior and lateral myocytes from nonfailing (control), DHF, and CRT dogs were studied with the whole-cell patch clamp. Quantitative polymerase chain reaction and Western blots were performed to measure steady state mRNA and protein levels. DHF significantly reduced the inward rectifier K(+) current (I(K1)), delayed rectifier K(+) current (I(K)), and transient outward K(+) current (I(to)) in both anterior and lateral cells. CRT partially restored the DHF-induced reduction of I(K1) and I(K) but not I(to), consistent with trends in the changes in steady state K(+) channel mRNA and protein levels. DHF reduced the peak inward Ca(2+) current (I(Ca)) density and slowed I(Ca) decay in lateral compared with anterior cells, whereas CRT restored peak I(Ca) amplitude but did not hasten decay in lateral cells. Calcium transient amplitudes were depressed and the decay was slowed in DHF, especially in lateral myocytes. CRT hastened the decay in both regions and increased the calcium transient amplitude in lateral but not anterior cells. No difference was found in Ca(V)1.2 (alpha1C) mRNA or protein expression, but reduced Ca(V)beta2 mRNA was found in DHF cells. DHF reduced phospholamban, ryanodine receptor, and sarcoplasmic reticulum Ca(2+) ATPase and increased Na(+)-Ca(2+) exchanger mRNA and protein. CRT did not restore the DHF-induced molecular remodeling, except for sarcoplasmic reticulum Ca(2+) ATPase. Action potential durations were significantly prolonged in DHF, especially in lateral cells, and CRT abbreviated action potential duration in lateral but not anterior cells. Early afterdepolarizations were more frequent in DHF than in control cells and were reduced with CRT.
Conclusions: CRT partially restores DHF-induced ion channel remodeling and abnormal Ca(2+) homeostasis and attenuates the regional heterogeneity of action potential duration. The electrophysiological changes induced by CRT may suppress ventricular arrhythmias, contribute to the survival benefit of this therapy, and improve the mechanical performance of the heart.
Figures






Comment in
-
Cardiac resynchronization therapy in dyssynchronous heart failure: zooming in on cellular and molecular mechanisms.Circulation. 2009 Mar 10;119(9):1192-4. doi: 10.1161/CIRCULATIONAHA.108.841544. Epub 2009 Feb 23. Circulation. 2009. PMID: 19237655 No abstract available.
References
-
- Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. - PubMed
-
- Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. - PubMed
-
- Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P, Wooley CF. Functional abnormalities in isolated left bundle branch block: the effect of interventricular asynchrony. Circulation. 1989;79:845–853. - PubMed
-
- Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF, Kass DA. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003;108:929–932. - PubMed
-
- Spragg DD, Akar FG, Helm RH, Tunin RS, Tomaselli GF, Kass DA. Abnormal conduction and repolarization in late-activated myocardium of dyssynchronously contracting hearts. Cardiovasc Res. 2005;67:77–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

